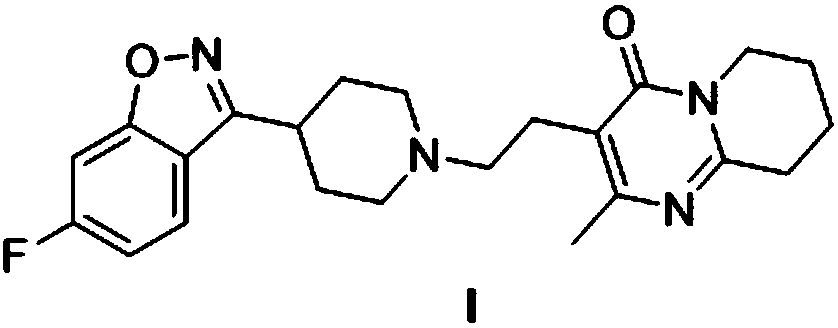
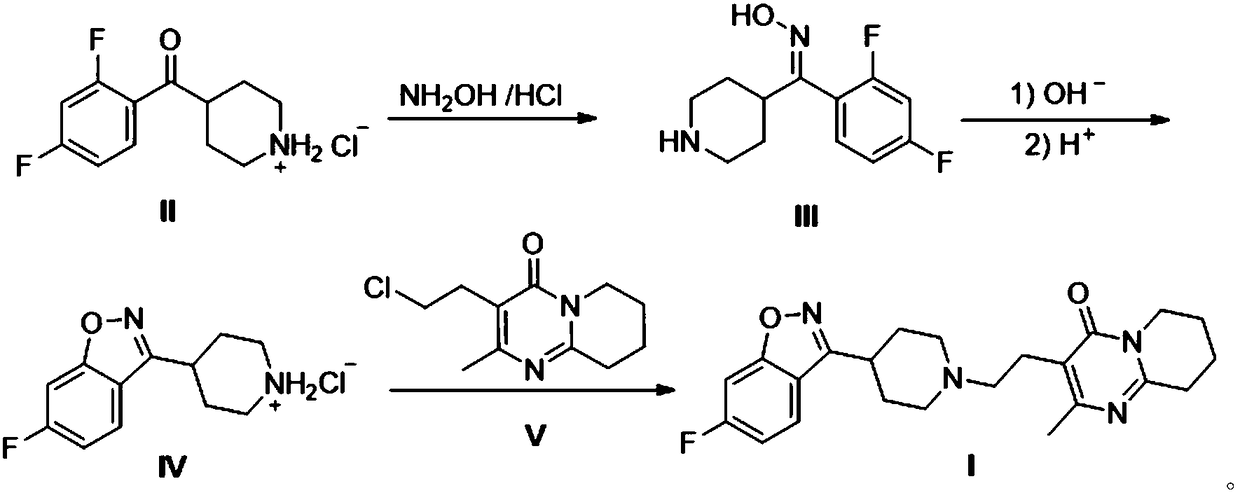
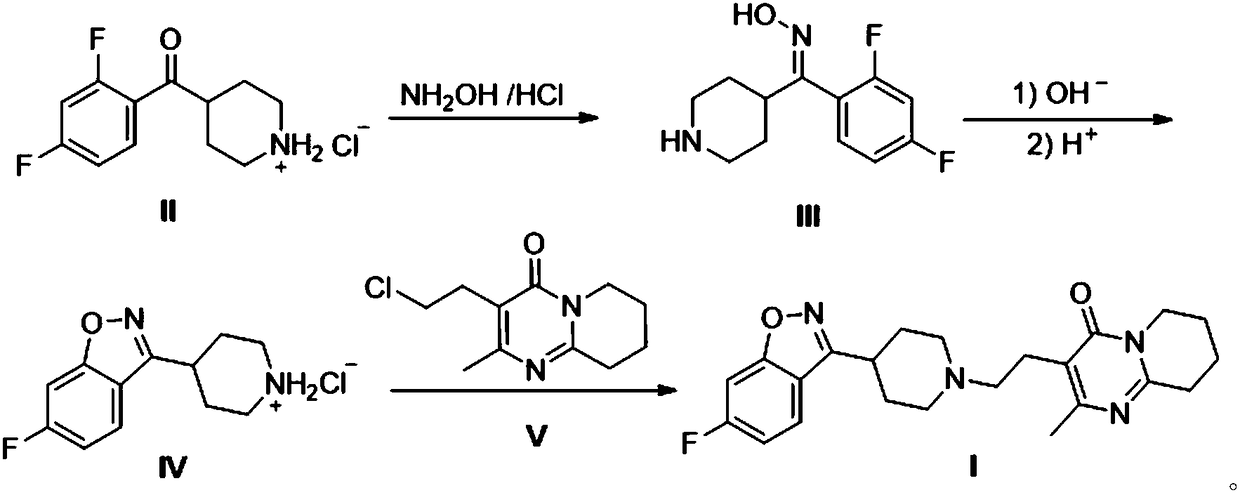
Preparation method of risperidone
A technology for risperidone and piperidinone hydrochloride, which is applied in the field of chemical drug intermediate preparation, can solve the problems of affecting the quality of raw materials, difficult to remove impurities, low total yield and the like, and achieves strong product market competitiveness, The effect of good industrialization and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of 2,4-difluorophenyl-4-piperidinemethanone oxime (Ⅲ).
[0023] In a 1000mL airtight four-necked reaction flask, add 52.3 grams of 2,4-difluorophenyl-4,-piperidine ketone hydrochloride (II), 13.9 grams of hydroxylamine hydrochloride, 19.8 grams of N-methylpiperidine and 500mL of ethanol, stirred and controlled the temperature at 60°C, reacted for 12 hours, stopped stirring, cooled to room temperature and filtered to obtain 24.8 grams of white solid 2,4-difluorophenyl-4-piperidinemethanone oxime (Ⅲ), yield 51.6 %.
Embodiment 2
[0024] Example 2: Preparation of 2,4-difluorophenyl-4-piperidinemethanone oxime (Ⅲ).
[0025] In a 1000mL sealed four-necked reaction flask, add 52.3 grams of 2,4-difluorophenyl-4,-piperidine ketone hydrochloride (II), 27.8 grams of hydroxylamine hydrochloride, 19.8 grams of N-methylpiperidine and Ethanol 500mL, stirring temperature control 60 ℃, reacted for 12 hours, stopped stirring, cooled to room temperature and filtered to obtain 36.7 grams of white solid 2,4-difluorophenyl-4,-piperidinemethanone oxime (Ⅲ), yield 76.3%.
Embodiment 3
[0026] Example 3: Preparation of 2,4-difluorophenyl-4-piperidinemethanone oxime (Ⅲ).
[0027] In a 1000mL airtight four-necked reaction flask, add 52.3 grams of 2,4-difluorophenyl-4-piperidine ketone hydrochloride (II), 13.9 grams of hydroxylamine hydrochloride, 39.6 grams of N-methylpiperidine and ethanol 500mL, stirred and controlled the temperature at 60°C, reacted for 12 hours, stopped stirring, cooled to room temperature and filtered to obtain 32.9 grams of white solid 2,4-difluorophenyl-4,-piperidinemethanone oxime (Ⅲ), yield 68.4 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com