Use of Novel Chimeric Antigen Receptor Modified T Cells for Cancer Therapy
A chimeric antigen receptor, a new type of technology, applied to genetically modified cells, cells modified by introducing foreign genetic material, receptors/cell surface antigens/cell surface determinants, etc., can solve unsatisfactory curative effects, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, chimeric antigen receptor preparation
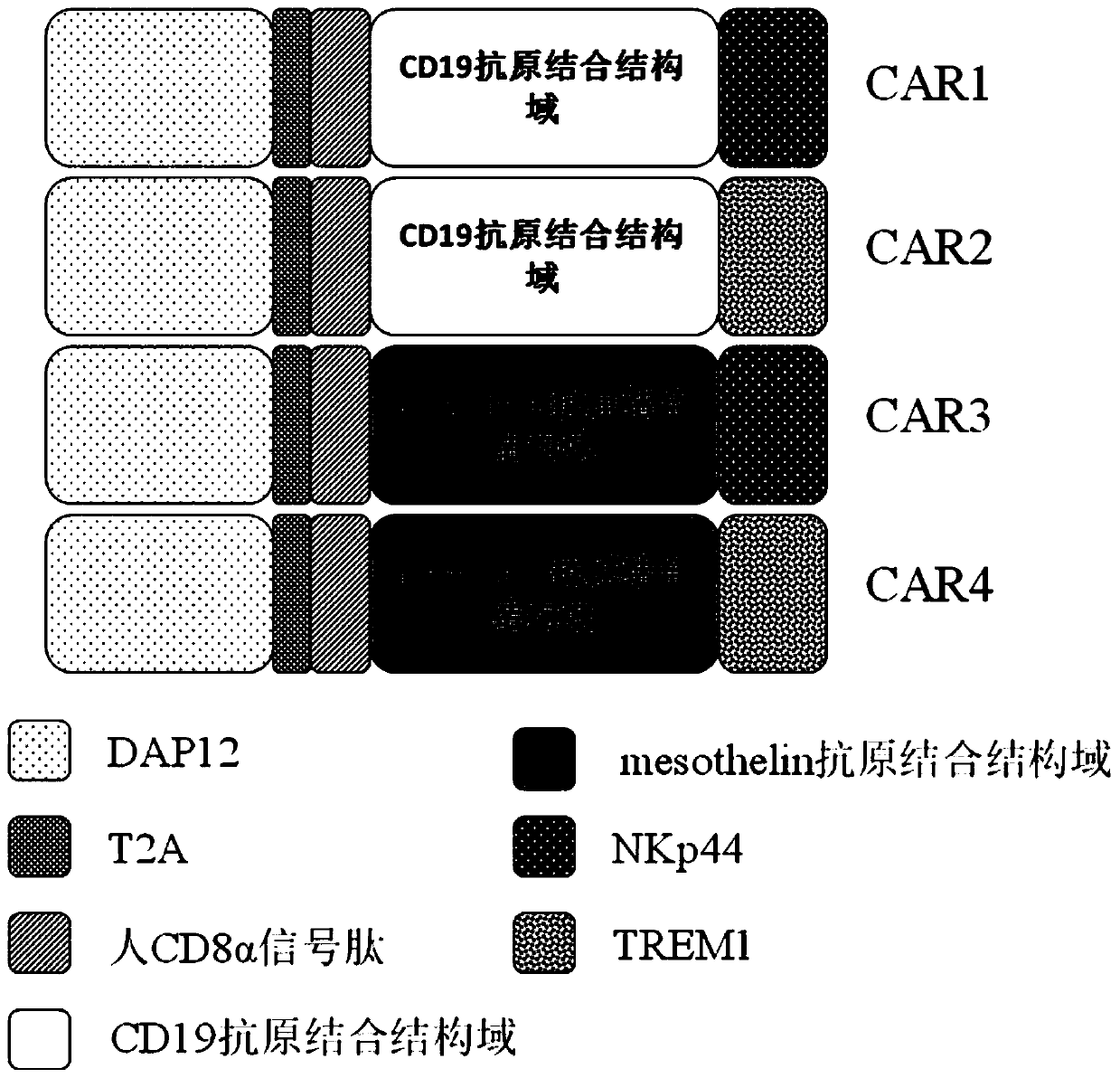
[0059] The present invention provides a novel chimeric antigen receptor. The chimeric antigen receptor of the present invention is composed of the second intracellular conduction domain-T2A-extracellular signal peptide-antigen binding domain-the first intracellular conduction domain in series. Therefore, viral vectors containing different combinations of stimulation signals need to be constructed separately. In this example, the single-chain antibody targeting CD19 and human mesothelin (mesothelin) are used as the unified extracellular recognition antigen structure, and the following four chimeric antigen receptors need to be constructed respectively ( figure 1 ):
[0060] DAP12-T2A-CD8α signal peptide-CD19 antigen-binding domain-NKp44(CAR1)
[0061] DAP12-T2A-CD8α signal peptide-CD19 antigen-binding domain-TREM1(CAR2)
[0062] DAP12-T2A-CD8α signal peptide-mesothelin antigen-binding domain-NKp44(CAR3)
[0063] ...
Embodiment 2
[0104] Embodiment 2, virus infection T cell
[0105] 1. Isolation and activation of T cells and virus infection
[0106] (1) Isolation of human peripheral blood mononuclear cells
[0107] Use a blood collection tube containing anticoagulant to collect about 10ml of peripheral blood, settle naturally at room temperature (18-25°C) for about 30min, collect the upper layer of plasma, and centrifuge the collected upper layer of plasma at 5000r / min for 10min at a volume ratio of 1:1 Add to the lymphocyte separation medium (purchased from Tianjin Haoyang Biological Products Technology Co., Ltd.), gradient centrifugation, 3000r / min, centrifugation for 30min, after centrifugation, the centrifuge tube is layered from top to bottom: the first layer is the plasma layer ; The second layer is the buffy coat layer of lymphocytes; the third layer is the transparent separation liquid layer; the fourth layer is the red blood cell layer. Aspirate the buffy coat of lymphocytes, wash twice with ...
Embodiment 3
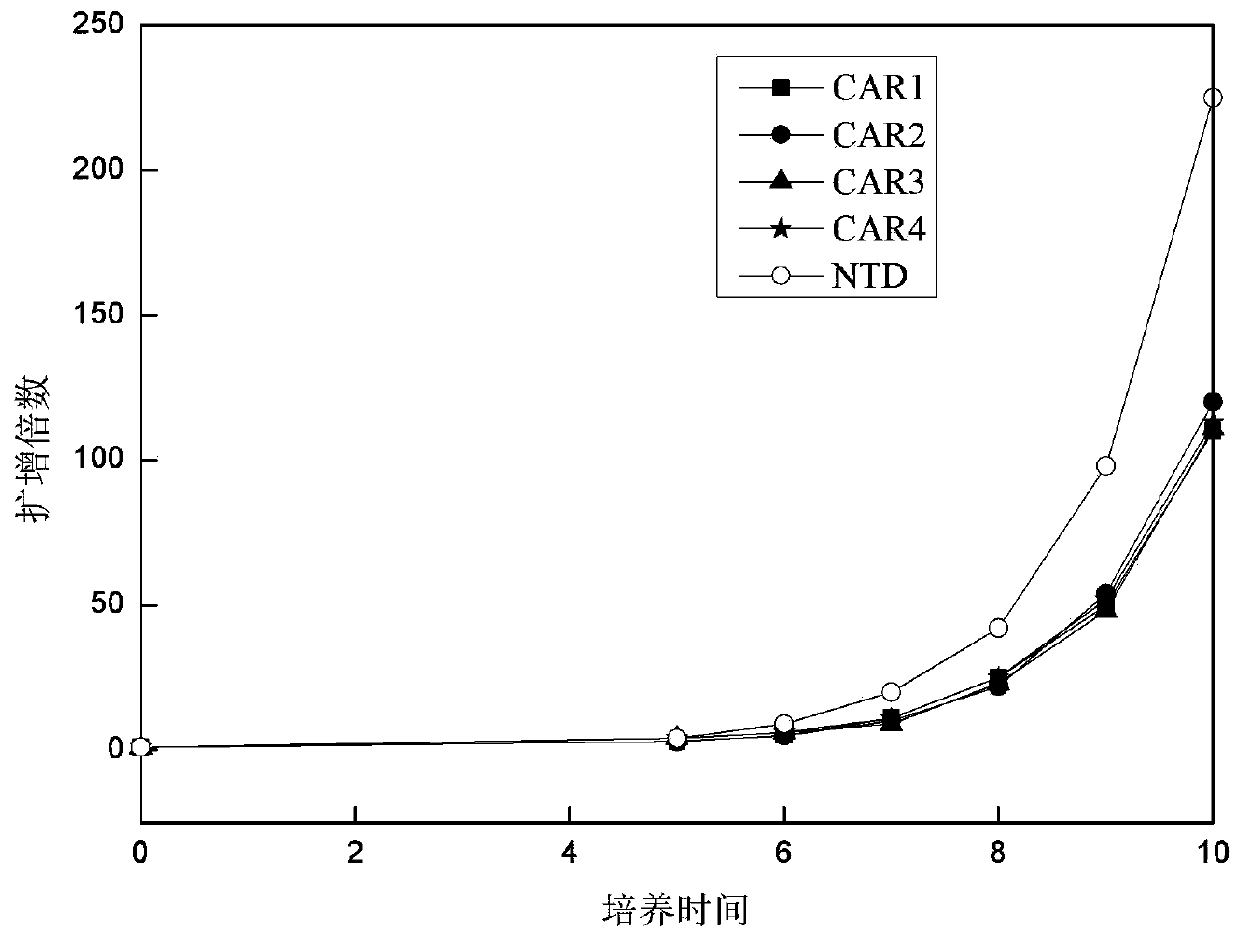
[0112] Example 3. Effect of Virus Infection of CAR-T Cells on Cell Proliferation
[0113]After each group of viruses infected T cells, the T cells were counted every 1-2 days with a complete medium containing 5% autologous plasma + 300 IU / ml recombinant human IL-2 + KBM581. Then observe the growth of T lymphocytes, the results are as follows: image 3 shown. The results showed that after the cells were infected with CAR-expressing viruses, they could still form a typical proliferating clonal group. By counting the cells and drawing the cell proliferation curve, it could be seen that CAR1, CAR2, CAR3, and CAR4 proliferated similarly, compared with T cells not infected with the virus ( image 3 Medium NTD) proliferative ability was slightly weaker.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com