Application of tazarotene to preparation of drugs used for treating hepatitis B virus infection
A technology of tazarotene and hepatitis B virus, applied in the field of medicine, can solve problems such as the inhibition of hepatitis B virus by tazarotene which has not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1HB
[0022] Embodiment 1 HBV infection system embodiment
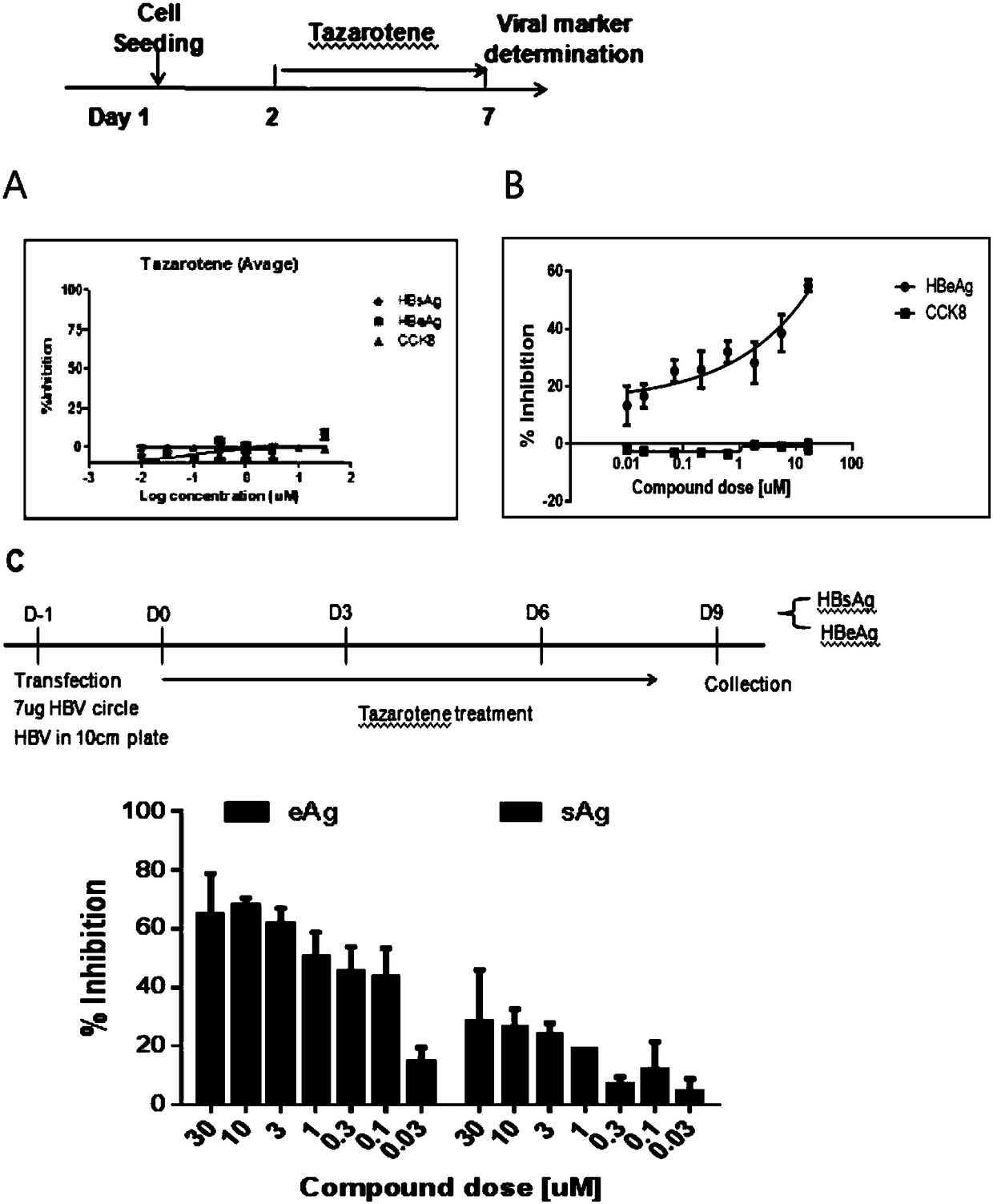
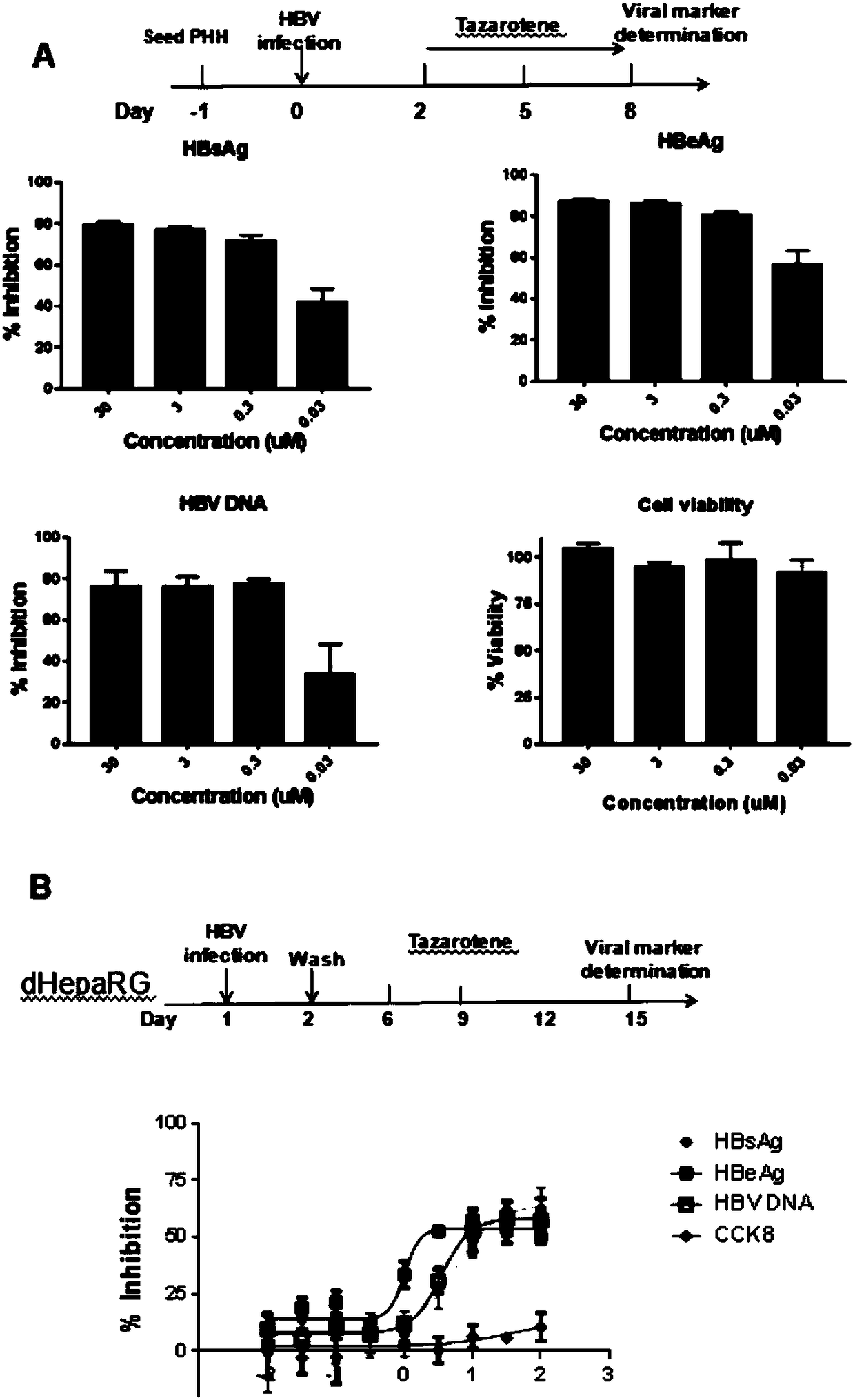
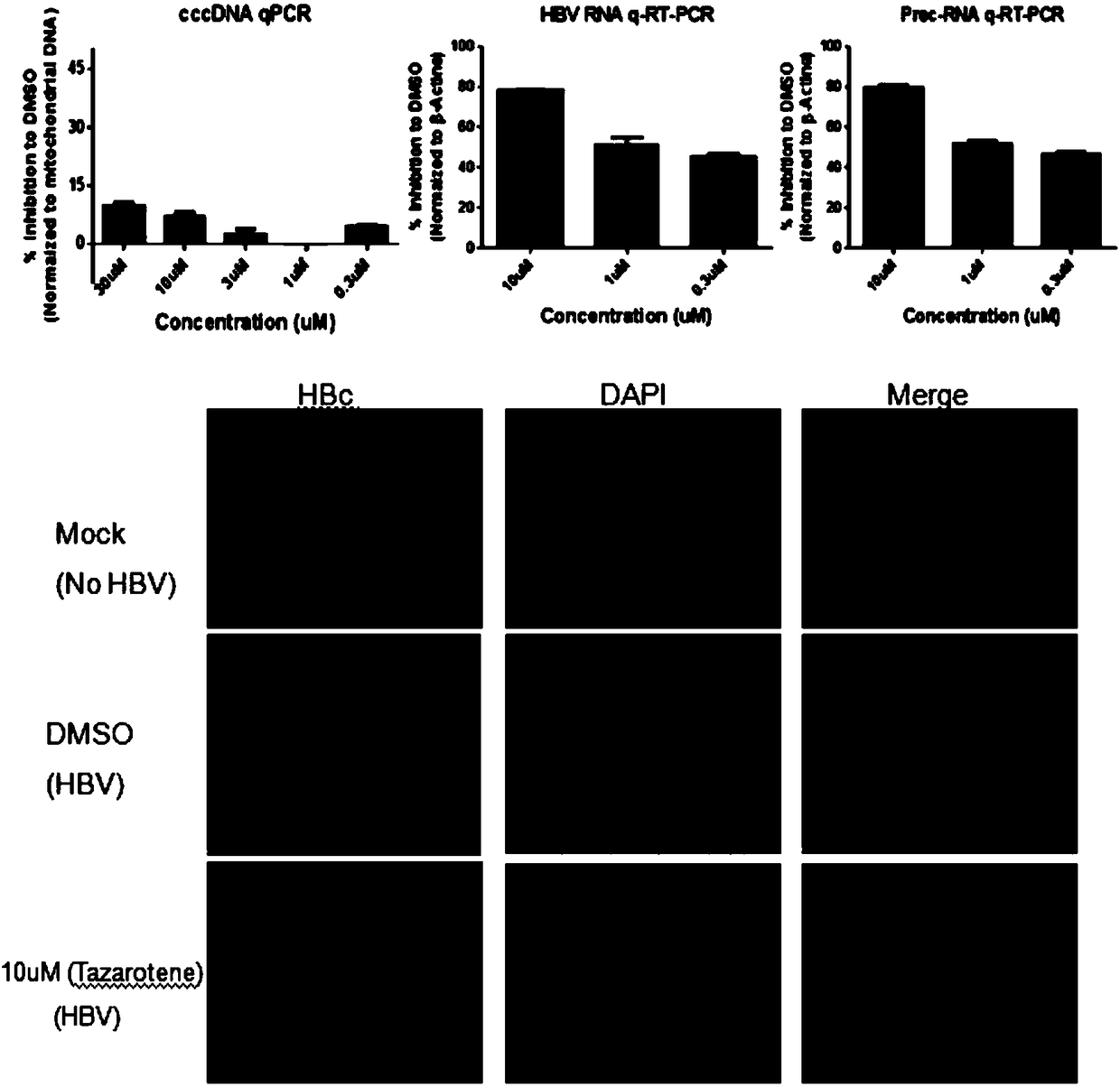
[0023] Treating PHH or HepaRG cells infected with hepatitis B virus particles with tazarotene inhibited the production of hepatitis B virus e antigen (HBeAg), hepatitis B surface antigen (HBsAg) and HBV DNA to varying degrees.
[0024] (1) HepaRG cell culture: HepaRG cell line is derived from terminally differentiated cells of human liver precursor cell line, which retains many characteristics of primary human hepatocytes, and can support HBV infection after differentiation; 1) Growth medium: 15 % fetal bovine serum, 100U / L penicillin, 0.1mg / L streptomycin, glutamine, 0.023IU / ml insulin, 4.7μg / ml hydrocortisone, 80μl / ml gentamicin; 2) Differentiation medium : Growth medium + 2% DMSO, need to continue to differentiate for more than two weeks for HBV infection;
[0025] (2) Cultivation of PHH cells: purchased from Shanghai Ruide Biology and cultured in a special commercial medium; it should be pointed out that in the researc...
Embodiment 2
[0031] Embodiment 2.HBV replicative cell line model embodiment
[0032] Test the inhibitory effect of tazarotene on HBV cell lines:
[0033] (1) Cultivation of the liver cell line Hep2.215: use DMEM / F12 culture medium (Gibco company, add 10% fetal bovine serum, 100U / ml penicillin, 100mg / ml streptomycin and 400mg / ml G418) in 5% CO 2 Constant temperature cultivation at 37°C under saturated water vapor environment;
[0034] (2) Cultivation of the liver cell line HepDES19: use DMEM / F12 culture medium (Gibco company, add 10% fetal bovine serum, 100U / ml penicillin, 100mg / ml streptomycin and 400mg / ml G418) in 5% CO 2Incubate at a constant temperature of 37°C in a saturated water vapor environment;
[0035] (3) In Hep2.215 cells and HepDES19 cells, different concentrations of tazarotene were added to the cells, and the cell supernatants were collected after 5 days of treatment to detect HBV antigen markers HBeAg or HBsAg by ELISA; the results showed that tazarotene was Ting was not...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com