Tesidolumab for use in the treatment of transplant rejection
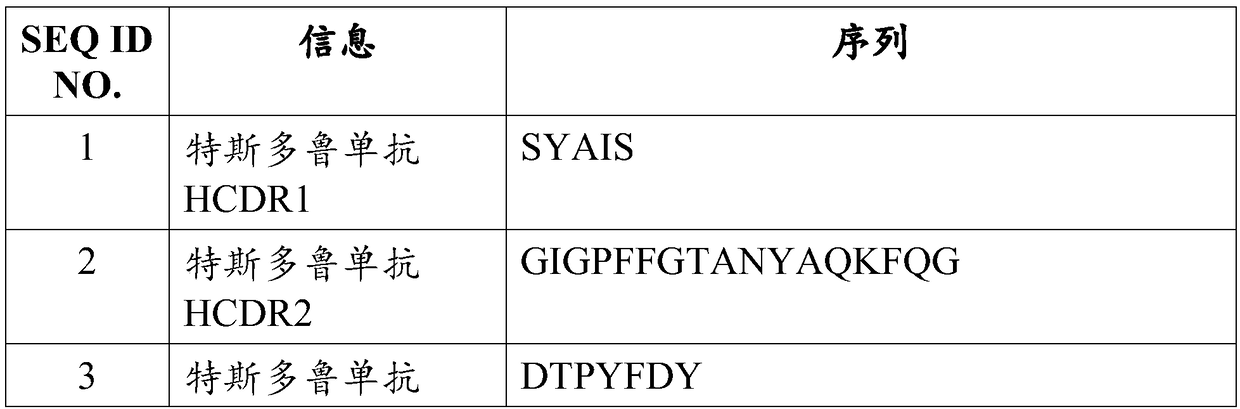
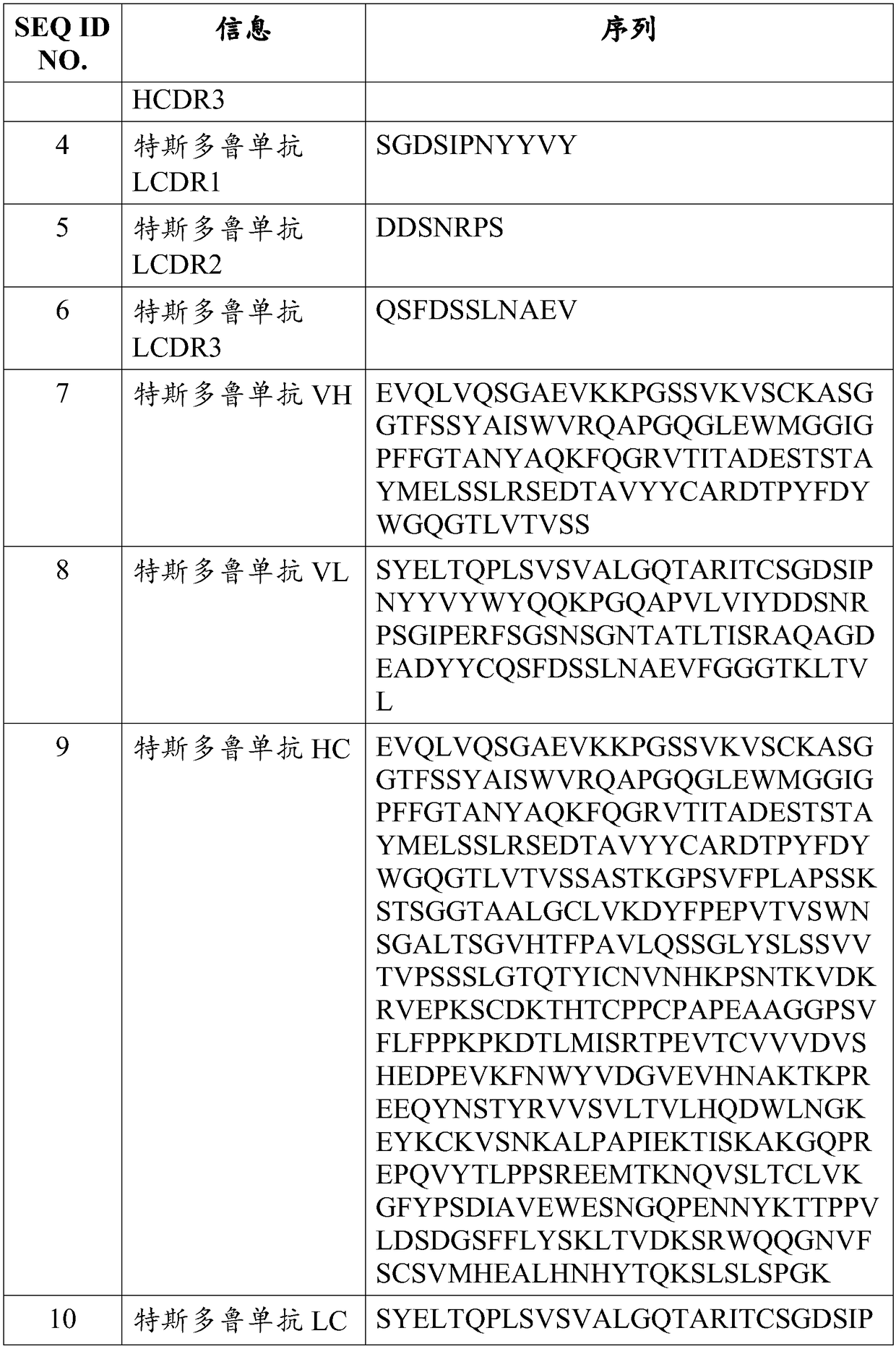
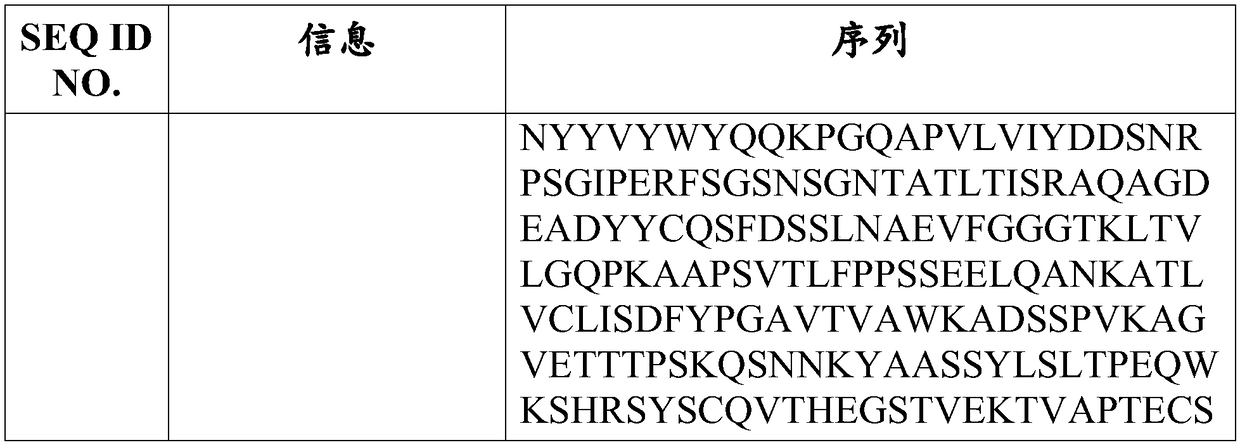
A transplant rejection and pre-transplant technology, applied in the direction of antibodies, specific peptides, antibody medical components, etc., can solve problems such as difficulties, increase the risk of infection with Neisseria meningitidis, and susceptibility to opportunistic infections.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0137] The relationship between serum concentration of total testolumab and serum complement activity was determined. Analysis of these data indicated that total testolumab concentrations below 55 μg / mL resulted in less than complete inhibition of serum complement activity.
[0138] Using modeling, the relationship between testolumab dose and exposure suggests that a dose of 20 mg / kg every two weeks is sufficient to ensure inhibition of complement activity. According to this model, less than 0.5% of patients had exposure values at trough levels below the limit of 55 μg / ml.
[0139] Based on the relationship between total serum concentrations of tesdolumab and serum complement activity, it has been found that total serum tesdolizumab concentrations <55 μg / mL result in less than complete inhibition of serum complement activity. Therefore, a minimum total serum concentration of tesidolumab of 55-100 μg / mL is sufficient to ensure inhibition of complement activity.
example 2
[0141] For the Phase 2 study of prevention of antibody-mediated rejection (AMR) after kidney transplantation, two cohorts of presensitized kidney transplant recipients (KTR) at high or intermediate risk of developing AMR will be recruited. Forty-eight KTRs will be enrolled according to immune risk as defined by their pre-existing donor-specific antibody concentration (DSA) and B-cell flow cytometry crossmatch (BFXM)-based functional assessment of immune risk. Both groups will receive the same treatment regimen with tesidolumab in addition to conventional immunosuppressive therapy and local pre- and post-transplant desensitization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com