Preparation method and application of 4-imidazolyl-containing glutaminyl cyclase inhibitor
A glutaminyl cyclase and imidazole-based technology, which is applied in the field of preparation of glutaminyl cyclase inhibitors, can solve the problems of cumbersome synthesis steps, single key pharmacophore structure, and limited molecular activity, and achieve High yield, simple and feasible process route, high activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
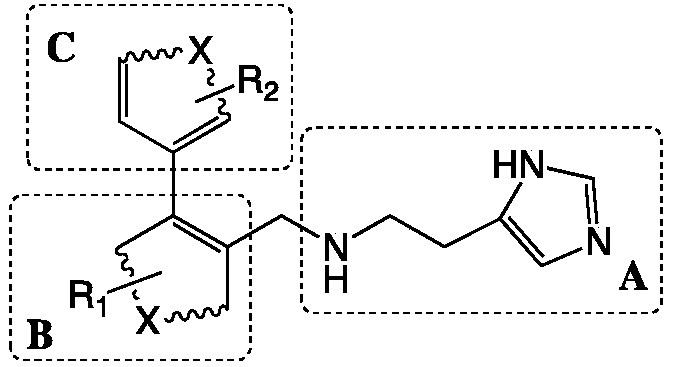
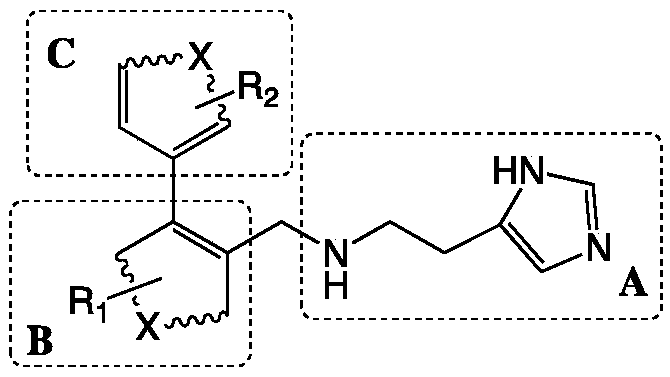
[0026] Wherein, the preparation method of the glutaminyl cyclase inhibitor containing 4-imidazolyl comprises steps:
[0027] with bromo substituents and R 1 The B unit raw material and with boronic acid group and R 2 Starting from the C unit raw material, the biphenyl intermediate of the B unit and the C unit coupling is prepared through the Suzuki coupling reaction;
[0028] Using the biphenyl intermediate coupled with the B unit and the C unit and 2-(1H-imidazol-4-yl)ethylamine as raw materials, the SN 2 The reaction prepares the glutaminyl cyclase inhibitor containing 4-imidazolyl;
[0029] The linked positions of the A unit and the C unit in the B unit are adjacent positions.
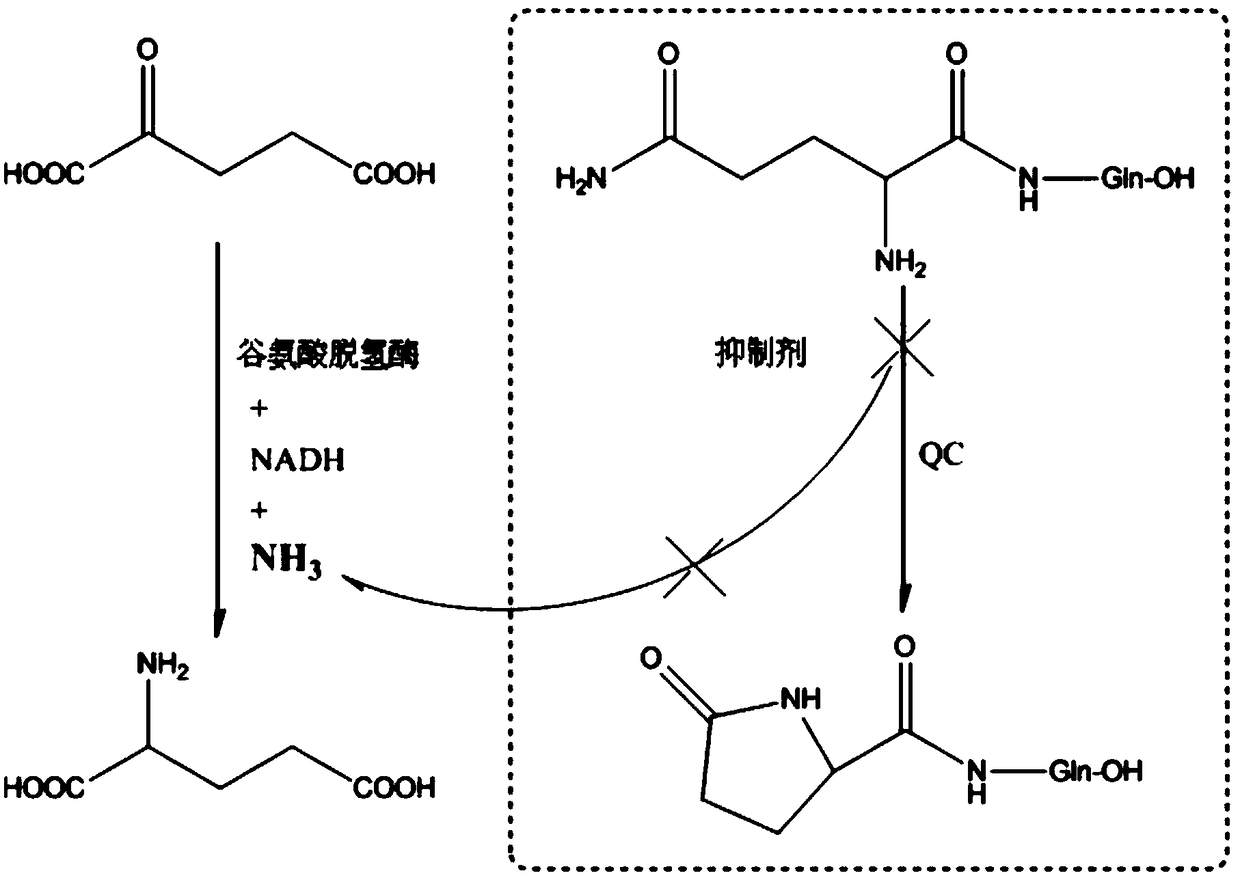
[0030] Specifically, since the existing chemical structural formula is The free N-H on the 1-imidazolyl pharmacophore on the glutaminyl cyclase inhibitor is difficult to form hydrogen bonds with the Asp248 amino acid residue in the active center of glutaminyl cyclase, which can only reduce the ...
Embodiment 1
[0044] The synthesis of N-([1,1'-biphenyl]-2-methyl)-2-(1H-imidazol-4-yl)ethyl-1-amine, its synthetic route is as follows:
[0045]
[0046] 1), the preparation of 2-(bromomethyl)-1,1'-biphenyl:
[0047] 2-Bromomethylbromobenzene (7.53 mmol, 1 equiv), phenylboronic acid (9.04 mmol, 1.2 equiv) and [1,1'-bis(diphenylphosphino)ferrocene]palladium(II) dichloride Dichloromethane complex (0.45mmol, 0.06 equivalent) was placed in a 50ml round bottom flask, and 10ml of dioxane and 10ml of 2mol / L K 2 CO 3 Solution, 100 ° C, reflux for 3h. Add saturated NaCl solution to quench the reaction, cool to room temperature, extract three times with ethyl acetate, wash once with saturated NaCl solution after combining, anhydrous NaCl 2 SO4 dried, and the product was collected by silica gel column chromatography with a yield of 79%.
[0048] 2), the preparation of N-([1,1'-biphenyl]-2-methyl)-2-(1H-imidazol-4-yl)ethyl-1-amine:
[0049] Dissolve 2-(bromomethyl)-1,1'-biphenyl (1.35mmol, 1 e...
Embodiment 2
[0051] N-(3',4,4',5'-tetramethoxy-[1,1'-biphenyl]-2-methyl)-2-(1H-imidazol-4-yl)ethyl-1 -The synthesis of amine, its synthetic route is as follows:
[0052]
[0053] 1), the preparation of 2-(bromomethyl)-3',4,4',5'-tetramethoxy-1,1'-biphenyl:
[0054] 2-Bromomethyl-4-methoxybromobenzene (6.49mmol, 1 equivalent), 3,4,5-trimethoxyphenylboronic acid (7.78mmol, 1.2 equivalents) and [1,1'-bis(di Phenylphosphine) ferrocene] dichloropalladium (II) dichloromethane complex (0.39mmol, 0.06 equivalents) is placed in a 50ml round bottom flask, and 10ml of dioxane and 10ml of 2mol / L K 2 CO 3 Solution, 100 ° C, reflux for 3h. Add saturated NaCl solution to quench the reaction, cool to room temperature, extract three times with ethyl acetate, wash once with saturated NaCl solution after combining, anhydrous NaCl 2 SO 4 After drying, the product was collected by silica gel column chromatography with a yield of 63%.
[0055] 2), N-(3',4,4',5'-tetramethoxy-[1,1'-biphenyl]-2-methyl)-2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com