Synthetic method for cinnoline compound and agricultural biological activity thereof
A synthetic method and compound technology, applied in botany equipment and methods, chemicals for biological control, biocides, etc., can solve the problems of uneconomical, high reaction cost, high metal residue, etc., and achieve short reaction time , Low catalyst dosage, simple reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
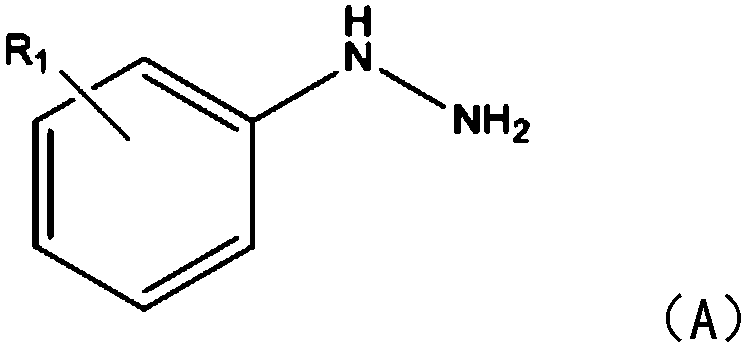
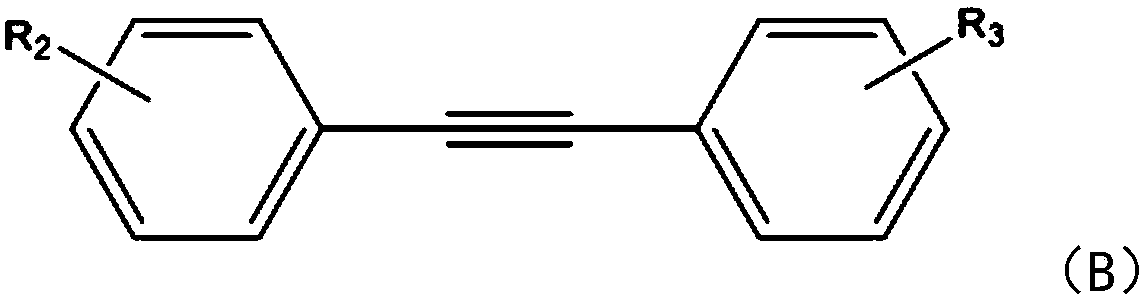
[0022] The embodiment of the present invention provides a kind of synthetic method of carnoline compound, comprises the following steps:
[0023] S1: Add phenylhydrazine compound and tolanyl acetylene compound respectively into the reaction container, under the action of copper sulfate and isopropanol, react for 1-2 hours under blue LED lighting at room temperature at 20-25°C;
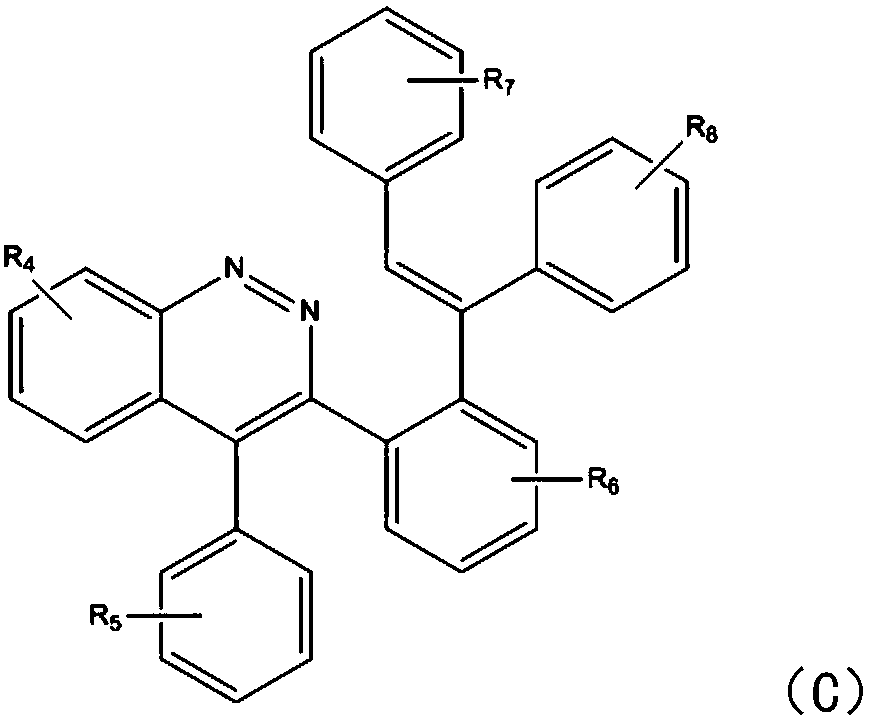
[0024] In this step, the cinnoline compound is synthesized by using the phenylhydrazine compound and the tolan compound. Specifically, copper sulfate reacts with phenylhydrazine to generate a five-membered ring intermediate, and the tolan compound inserts the five-membered ring In the next step, another molecule of benzyne compound is inserted into the intermediate of the previous step to generate the target product. What needs to be explained here is that the reaction in this step does not need to be heated, and it can be reacted under light conditions. The amount of copper sulfate catalyst is extreme...
Embodiment 1
[0040] Add 1mmol, 2mmol, 0.003mmol copper sulfate and 2ml isopropanol to the reaction vessel respectively, and react for 1 hour under the blue 5W LED light at room temperature 20-25°C; after the reaction, add water and dichloromethane to extract , the organic layers were combined and then the organic solvent was removed to give the following C1 compound:
[0041]
[0042] Carry out nuclear magnetic spectrum analysis to above-mentioned yellow solid, data is as follows:
[0043] 1 H NMR (500MHz, CDCl 3 )δ=8.53(d, J=8.5Hz, 1H), 7.74(s, 1H), 7.61(d, J=8.2Hz, 1H), 7.58(d, J=7.0Hz, 1H), 7.43-7.31( m,6H),7.28(d,J=4.1Hz,1H),7.11(d,J=7.0Hz,2H),7.06-7.00(m,3H),6.96(t,J=7.4Hz,1H), 6.92-6.87(m, 2H), 6.85(t, J=7.6Hz, 2H), 6.56(d, J=7.3Hz, 2H), 6.23(s, 1H);
[0044] 13 C NMR (125MHz, CDCl 3 )δ=153.9, 149.0, 144.4, 141.8, 139.9, 137.1, 137.0, 133.6, 133.0, 132.1, 131.2, 130.7, 130.5, 129.7, 129.6, 129.1, 128.3, 128.1, 127.8, 120.6, 12.7 ,125.0,124.7;
[0045] After identification, t...
Embodiment 2
[0047] Add 1mmol, 2mmol, 0.003mmol copper sulfate and 2ml isopropanol to the reaction vessel respectively, and react for 1 hour under the blue 5W LED light at room temperature 20-25°C; after the reaction, add water and dichloromethane to extract , combining the organic layers and then removing the organic solvent yields the following C2 compound:
[0048]
[0049] Carry out nuclear magnetic spectrum analysis to above-mentioned yellow solid, data is as follows:
[0050] 1 H NMR (500MHz, CDCl 3 )δ=7.54(td,J=8.0,5.1Hz,1H),7.47-7.31(m,8H),7.28(s,1H),7.10(d,J=7.8Hz,2H),7.05(dd,J =5.1,1.5Hz,3H),7.00(t,J=7.4Hz,1H),6.89(t,J=7.6Hz,4H),6.58(d,J=8.1Hz,2H),6.25(s,1H );
[0051] 13 C NMR (125MHz, CDCl 3 )δ=158.6 (d, J=263.0Hz), 154.9, 144.4, 141.9, 140.3, 140.2, 139.9, 137.2, 136.7, 133.4, 132.5, 132.0, 131.4, 130.8, 130.7, 130.6, 129.8, 129.4, 128 ,127.6,127.1,127.0,126.6,126.5,121.0,113.2;
[0052] After identification, the spectral data corresponded to the structural formula,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com