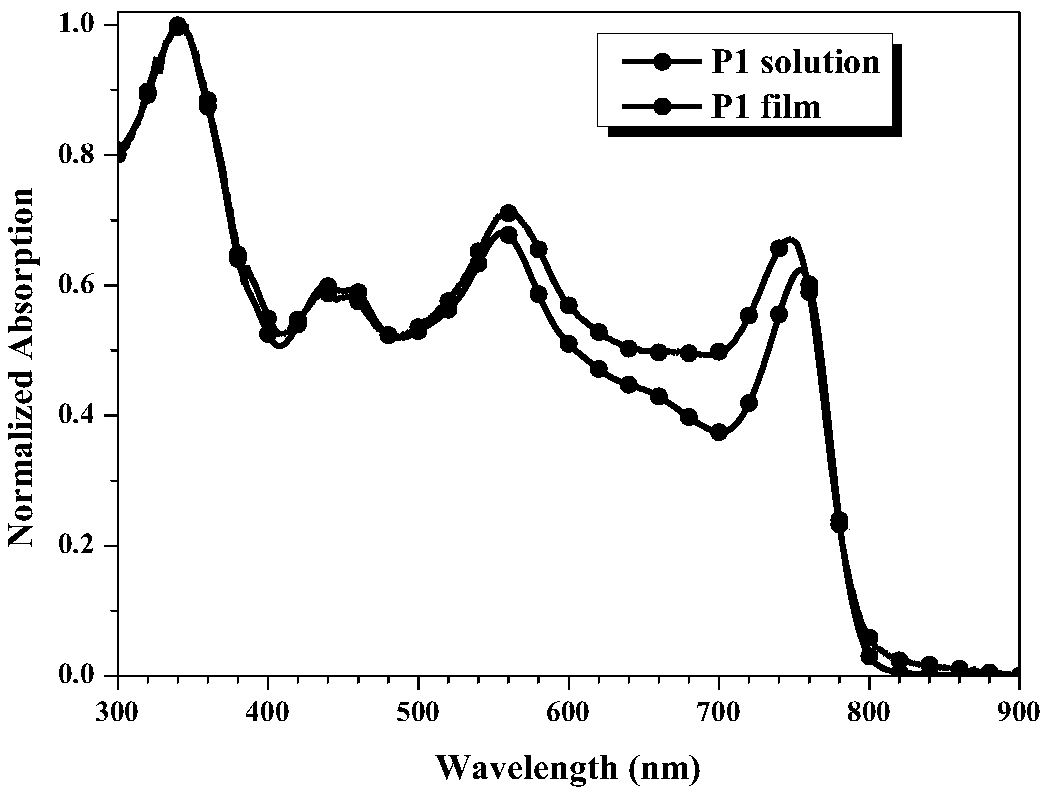
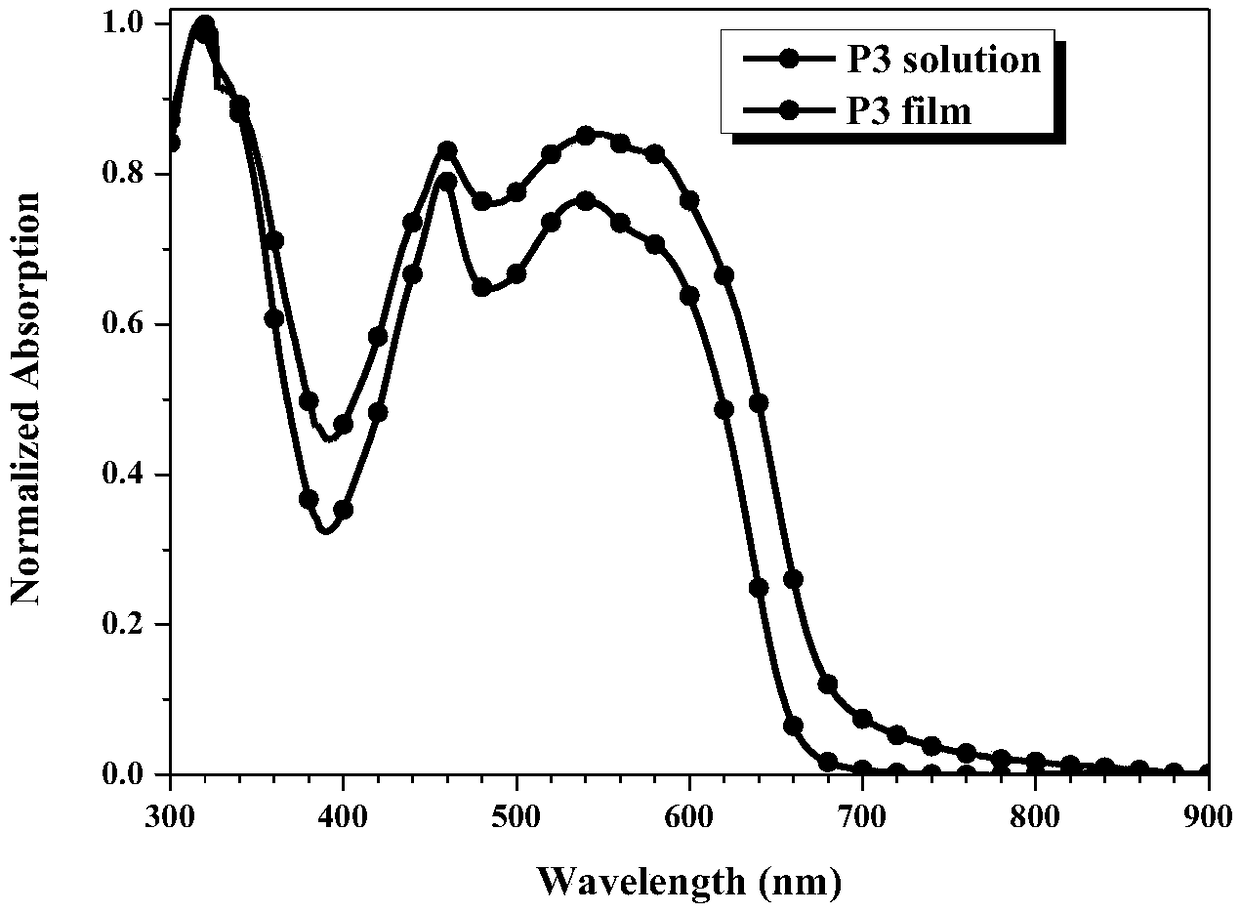
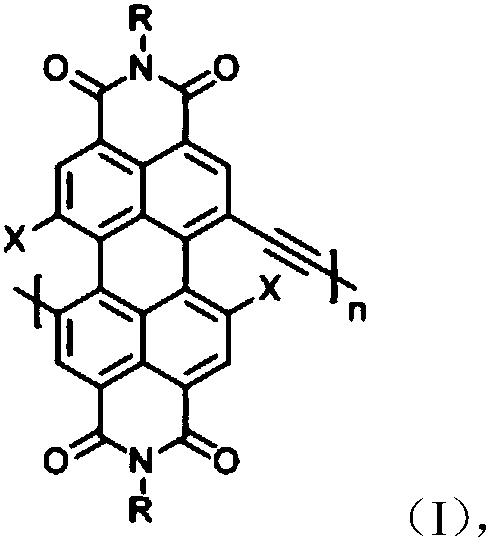
Perylene bisimide polymer as well as preparation method and application thereof
A perylene imide and polymer technology, applied in the field of polymer synthesis, can solve the problems of reducing electron mobility, reducing electron mobility, affecting device performance, etc., to achieve improved device performance, high fill factor, and high electron mobility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A perylene imide polymer, the reaction scheme is shown in formula III, and its preparation method specifically includes the following steps:
[0033] Take bis(trimethyltin)acetylene (82mg, 0.23mmol) and N,N'-bis(1-pentylhexyl)-1,7-dibromo-perylenediimide (200mg, 0.23mmol), three (dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 , 4.3mg, 4.7μmol) and tri-o-tolylphosphine (P(o-tol) 3 , 5.2mg, 17μmol) in a 25mL single-necked flask, after nitrogen pumping 3 times to remove the oxygen in the system, use a syringe to inject 5mL of toluene into the above reaction system under the protection of nitrogen, then raise the temperature to 120°C, and stir vigorously for 48h; Then the obtained polymer product system is subjected to end-capping treatment, and 100 mg of 2-tributylstannylthiophene is injected into the obtained product system under the protection of nitrogen for 12 hours, and then 200 mg of 3-bromothiophene is injected into the system for 12 hours, and then the crude prod...
Embodiment 2
[0036] A perylene imide polymer, the reaction scheme is shown in formula IV, and its preparation method specifically includes the following steps:
[0037] Take bis(trimethyltin)acetylene (136.6mg, 0.39mmol) and N,N'-bis(2-ethylhexyl)-1,7-dibromo-6,12-difluoroperylenediimide ( 300mg, 0.39mmol), tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 , 4.5mg, 4.9μmol) and tri-o-tolylphosphine (P(o-tol) 3, 5mg, 16μmol) in a 25mL single-necked flask, after nitrogen pumping 3 times to remove the oxygen in the system, inject 6mL of toluene into the reaction system with a syringe under the protection of nitrogen, raise the temperature to 120°C, stir vigorously for 48h, and then the obtained The polymer product system is end-capped, and 100mg of 2-tributylstannylthiophene is injected into the system under the protection of nitrogen for 12 hours, and then 200mg of 3-bromothiophene is injected into the system for 12 hours, and then the crude product is precipitated with methanol The cru...
Embodiment 3
[0040] A perylene imide polymer, the reaction scheme is shown in formula V, and its preparation method specifically includes the following steps:
[0041] Take bis(trimethyltin)acetylene (71mg, 0.2mmol) and N,N'-bis(1-pentylhexyl)-1,7-dibromo-6,12-difluoro-perylenediimide ( 180mg, 0.2mmol), tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 , 4.2mg, 4.6μmol) and tri-o-tolylphosphine (P(o-tol) 3 , 4.8mg, 16μmol) in a 25mL single-necked flask, after nitrogen pumping 3 times to remove the oxygen in the system, inject 4mL of toluene into the reaction system with a syringe under the protection of nitrogen, raise the temperature to 120°C, stir vigorously for 48h, and then The obtained polymer product system was subjected to end-capping treatment, and 100 mg of 2-tributylstannylthiophene was injected into the system under the protection of nitrogen for 12 hours, and then 200 mg of 3-bromothiophene was injected into the system for 12 hours, and then the crude product was precipitat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com