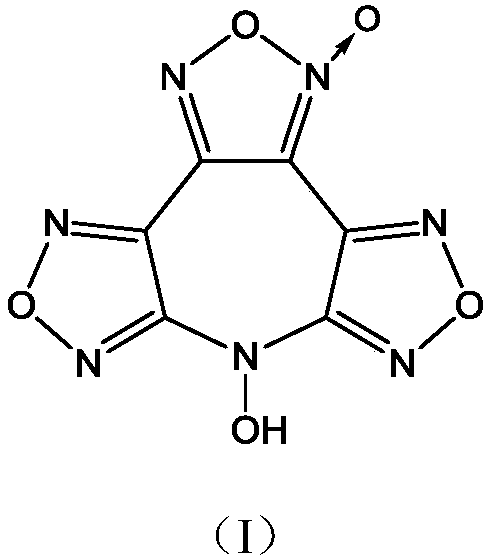
7-hydroxy difurazan and furoxan azacycloheptene compound
A technology of furoxan and heterocycloheptene, applied in the direction of organic chemistry, can solve problems such as low detonation velocity and pressure, poor oxygen balance, and low compound density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
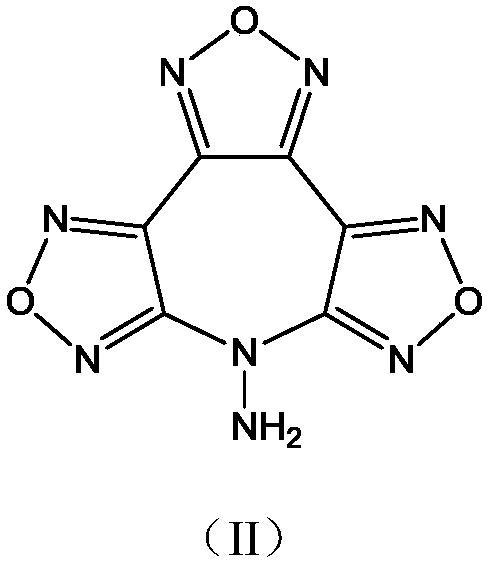
[0017] Synthesis of 7-Hydroxybisfurazanofurazanoazepine
[0018] Under stirring, at a temperature of -15°C, (4.5g, 15mmol) 3,4-bis(4'-nitrofurazan-3'-yl)furazan was added to anhydrous 30mL acetonitrile, and slowly added dropwise (3.0g, 45mmol) The mass fraction was 50% hydroxylamine aqueous solution, reacted for 45min, and filtered to obtain 1.8g of 7-hydroxybisfurazanofurazanoxepepene, with a yield of 44.7% and a purity of 98.8%.
[0019] Structure Identification:
[0020] Infrared Spectrum: IR(KBr,cm -1 ), υ: 3282, 2087, 2454, 1658, 1637, 1608, 1588, 1555, 1528, 1482, 1440, 1395, 1355, 1173, 1054, 996, 969, 939, 851, 797, 736;
[0021] NMR spectrum: 1 H NMR (DMSO-d 6 ,500MHz), δ: 7.81; 13 C NMR (DMSO-d 6 ,125MHz), δ: 155.08, 154.68, 144.98, 135.72, 133.66, 105.96;
[0022] Elemental analysis: Molecular formula C 6 HN 7 o 5
[0023] Theoretical value: C 28.70, H 0.40, N 39.04
[0024] Measured values: C 28.31, H 0.45, N 38.64;
[0025] The above structural identi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com