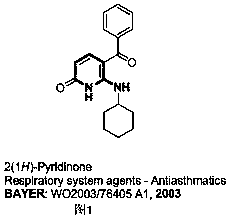
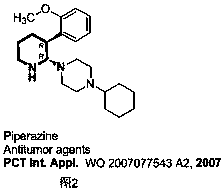
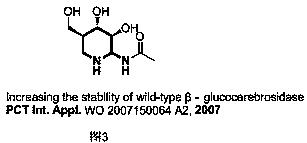Method for preparing 2-amino-3-methylene-1,2,3,6-tetrahydropyridine derivatives
A technology of tetrahydropyridine and tetrahydropyridine, which is applied in the direction of organic chemistry, can solve the problems of difficult operation, industrial application limitation, and low yield, and achieve the effects of stable performance, high yield, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] N-allyl-4-methyl-N-(3-methylene-5-phenyl-1-p-toluenesulfonyl-1,2,3,6-tetrahydropyridine)benzenesulfonamide: Figure 8
[0047] In a 25 mL test tube reactor, exchange the air with nitrogen 3 times. Substrate 1a (0.2 mmol, 49.7 mg), 2c (0.2 mmol, 82.7 mg), Pd 2 (dba) 3 (0.01 mmol, 9.1 mg) were weighed into the reaction tube in turn, evacuated to change nitrogen, and dioxane (2 mL) was added under nitrogen atmosphere, and 56 μL triethylamine was added to the pipette. Heat the reaction system to 80 o C, react for 5 hours. After the reaction was detected by TLC, the system was cooled to room temperature. Directly add silica gel, and spin dry column chromatography to obtain brown solid 3ca (50%). 1 H NMR (400 MHz, CDCl 3 ): δ 7.68 (d, J = 8.4 Hz,1H), 7.64 (d, J =8.0 Hz, 2H), 7.35-7.28 (m, 9H), 6.89 (s, 1H), 5.70-5.60 (m, 1H), 5.32 (s,1H), 5.14 (dd, J = 11.2, 1.2 Hz, 1H), 5.11-5.10 (m, 1H), 4.15 (s, 2H), 3.89(s, 2H), 3.76 (d, J = 6.4 Hz, 2H), 2.42 (s, 3H), 2.40...
Embodiment 2
[0049] N-allyl-N-(5-ethyl-3-methylene-1-p-toluenesulfonyl-1,2,3,6-tetrahydropyridine)-4-methylbenzenesulfonamide: Figure 9
[0050] In a 25 mL test tube reactor, exchange the air with nitrogen 3 times. Substrate 1a (0.2 mmol, 49.7 mg), 2d (0.2 mmol, 73.0 mg), Pd 2 (dba) 3 (0.01 mmol, 9.1 mg) were weighed into the reaction tube in turn, evacuated to change nitrogen, and dioxane (2 mL) was added under nitrogen atmosphere, and 56 μL triethylamine was added to the pipette. Heat the reaction system to 80 o C, react for 5 hours. After the reaction was detected by TLC, the system was cooled to room temperature. Silica gel was directly added, and column chromatography was spin-dried to obtain 3da (71%) as a brown solid. 1 H NMR (400 MHz, CDCl 3 ): δ 7.42 (d, J = 8.0 Hz 2H), 7.61 (d, J = 8.0 Hz, 2H), 7.28 (d, J = 8.0 Hz, 4H), 6.31 (s, 1H), 5.67-5.57 (m, 1H),5.15 (s, 1H), 5.12-5.07 (m, 2H), 3.75 (s, 2H), 3.71 (d, J = 6.4 Hz, 2H), 3.63(s, 2H), 3.95 (s, 2H), 2.42 (s, 3H),...
Embodiment 3
[0052] N-allyl-4-methyl-N-(3-methylene-5(p-tolyl)-1-toluenesulfonyl-1,2,3,6-tetrahydropyridine)-4-benzene Sulfonamide: Figure 10
[0053] In a 25 mL test tube reactor, exchange the air with nitrogen 3 times. Substrate 1a (0.2 mmol, 49.7 mg), 2e (0.2 mmol, 85.5 mg), Pd 2 (dba) 3 (0.01 mmol, 9.1 mg) were weighed into the reaction tube in turn, evacuated to change nitrogen, and dioxane (2 mL) was added under nitrogen atmosphere, and 56 μL triethylamine was added to the pipette. Heat the reaction system to 80 o C, react for 5 hours. After the reaction was detected by TLC, the system was cooled to room temperature. Silica gel was added directly, and column chromatography was spin-dried to obtain brown solid 3ea (43%). 1 H NMR (400 MHz, CDCl 3 ): δ 7.67 (d, J = 8.4 Hz, 2H), 7.64 (d, J = 8.0 Hz, 2H), 7.31 (d, J = 8.0 Hz, 2H), 7.28 (d, J = 8.4 Hz, 2H), 7.22 (d, J = 8.0 Hz,2H), 7.15 (d, J = 7.6 Hz, 2H), 6.85 (s, 1H), 5.70-5.60 (m, 1H), 5.31(s, 1H), 5.15-5.09 (m, 2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com