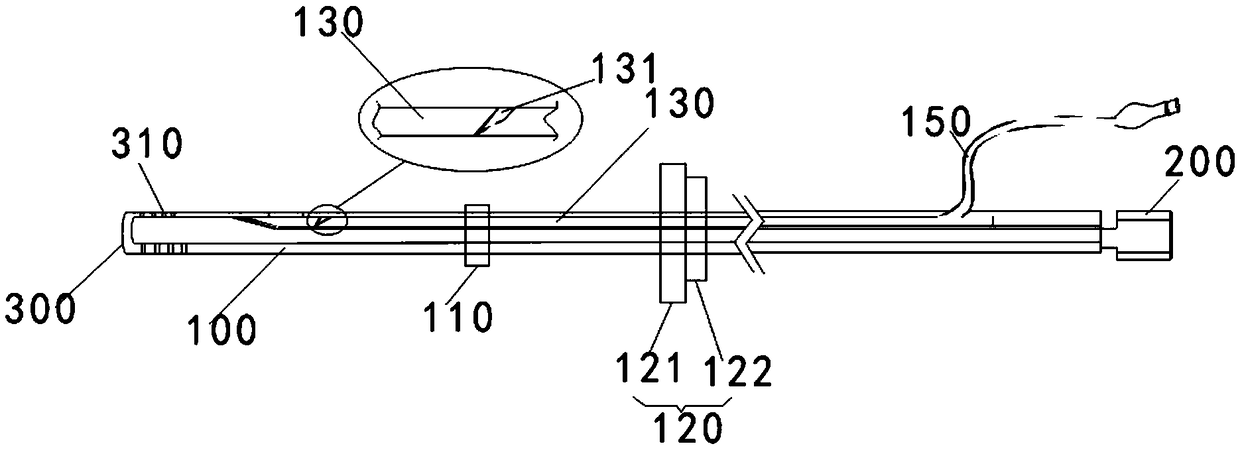
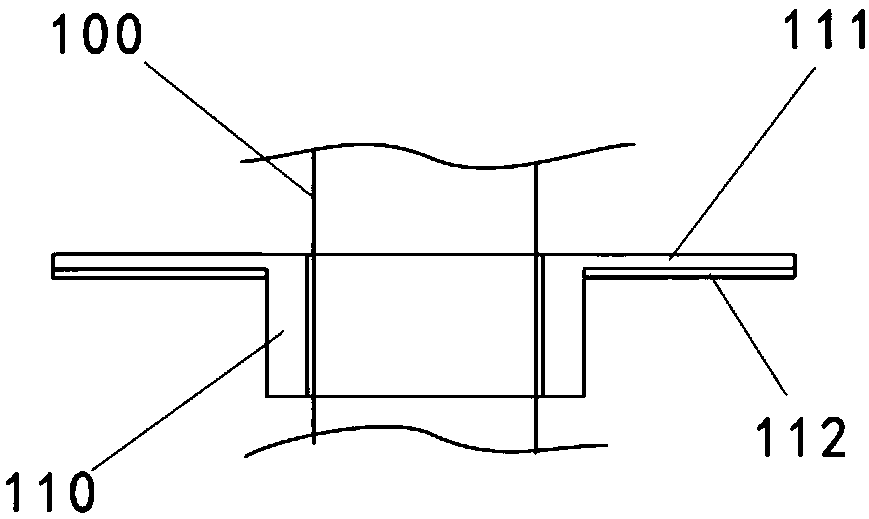
A safe and effective anti-blocking and anti-injury thoracic cavity drainage tube
An anti-injury and drainage tube technology, applied in the field of chest drainage tube, can solve the problems of patient suffering, discomfort and pain of patients, and achieve the effects of reducing pain, alleviating pain and preventing friction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Step a, respectively pulverizing the aloe, purslane, and myrobalan at 45°C, and filtering to obtain the corresponding filtrates A, B, and C respectively;
[0064] Step b, soak the filtrates A, B, and C obtained above in ethanol at 60° C. for 3 hours, remove the upper layer, and obtain the aloe extract, purslane extract, and myrobalan extract respectively, and correspondingly Denote as extract A, extract B and extract C;
[0065] Step c, after mixing 10 parts of a-cyanoacrylate and 3 parts of glycerin at 80°C, then adding 3 parts of extract A, stirring and mixing for 2 hours to form a mixture M1;
[0066] Step d. Mix 2 parts of carboxymethyl cellulose, 0.5 part of polyvinyl alcohol, 1 part of extract B and 1 part of extract C at 60°C-80°C, and stir for 2 hours to form a mixture M2;
[0067] In step e, the mixture M1 and the mixture M2 are combined, treated with ultraviolet rays for 2-3 hours, and cooled to room temperature to obtain the medical glue.
Embodiment 2
[0069] Step a, respectively pulverizing the aloe, purslane, and myrobalan at 45°C, and filtering to obtain the corresponding filtrates A, B, and C respectively;
[0070] Step b, soak the filtrates A, B, and C obtained above in ethanol at 60° C. for 3 hours, remove the upper layer, and obtain the aloe extract, purslane extract, and myrobalan extract respectively, and correspondingly Denote as extract A, extract B and extract C;
[0071] Step c. After mixing 12 parts of a-cyanoacrylate and 4 parts of glycerin at 80°C, add 4 parts of extract A, stir and mix for 2 hours to form a mixture M1;
[0072] Step d, after mixing 2 parts of carboxymethyl cellulose, 1 part of polyvinyl alcohol, 2 parts of extraction solution B, and 2 parts of extraction solution C at 80°C, and stirring for 2 hours, a mixture M2 was formed;
[0073] In step e, the mixture M1 and the mixture M2 are combined, treated with ultraviolet rays for 2 hours, and cooled to room temperature to obtain the medical glue....
Embodiment 3
[0075] Step a, respectively pulverizing the aloe, purslane, and myrobalan at 45°C, and filtering to obtain the corresponding filtrates A, B, and C respectively;
[0076] Step b, soak the filtrates A, B, and C obtained above in ethanol at 60° C. for 3 hours, remove the upper layer, and obtain the aloe extract, purslane extract, and myrobalan extract respectively, and correspondingly Denote as extract A, extract B and extract C;
[0077] Step c. After mixing 15 parts of a-cyanoacrylate and 6 parts of glycerin at 80°C, add 5 parts of extract A, stir and mix for 2 hours to form a mixture M1;
[0078] Step d. Mix 3 parts of carboxymethyl cellulose, 1 part of polyvinyl alcohol, 2 parts of extract B, and 2 parts of extract C at 80° C., and stir for 2 hours to form a mixture M2;
[0079] In step e, the mixture M1 and the mixture M2 are combined, treated with ultraviolet rays for 2 hours, and cooled to room temperature to obtain the medical glue.
[0080] The chest drainage device pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com