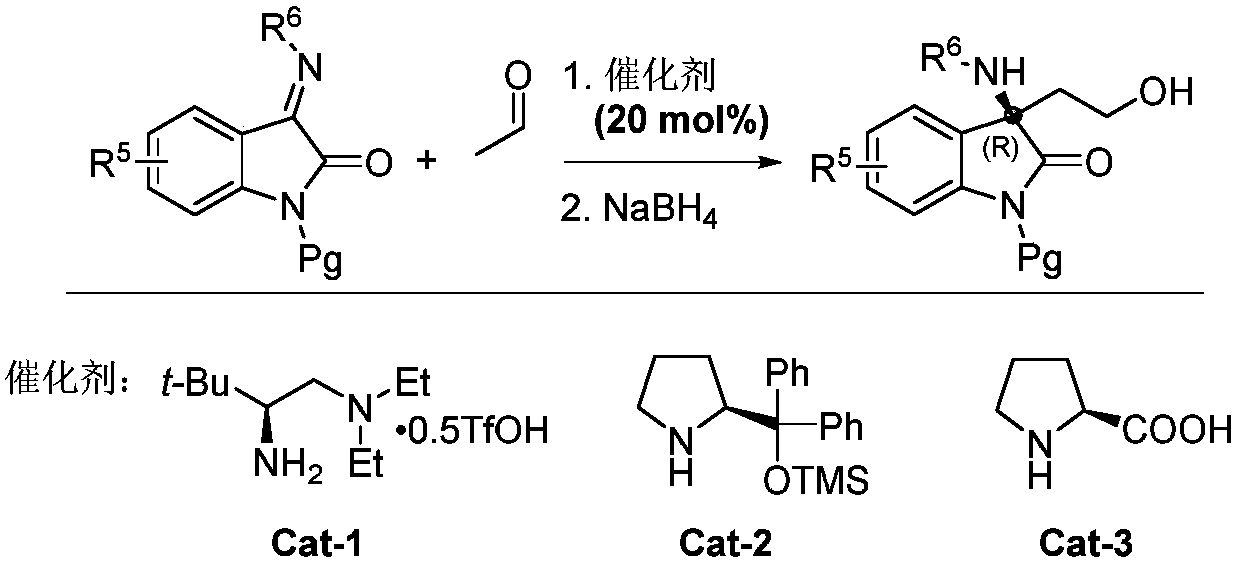
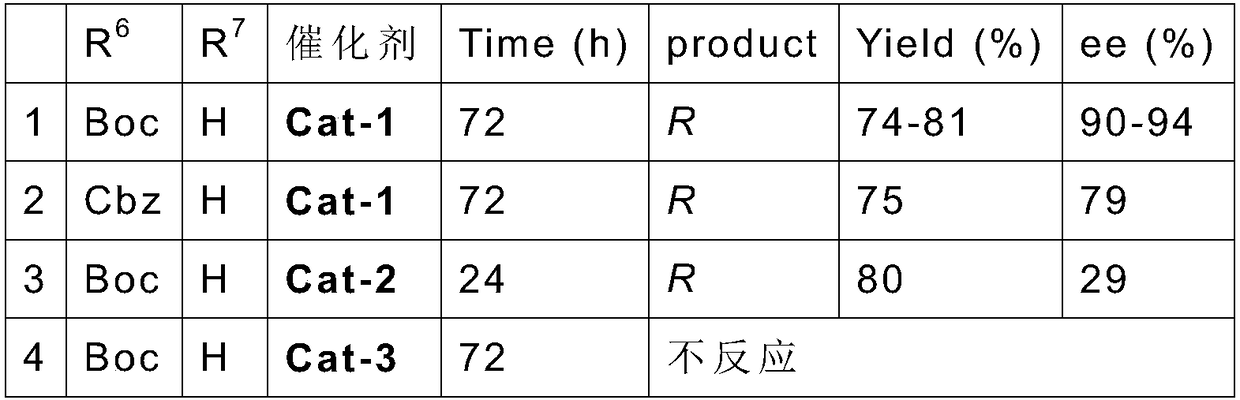
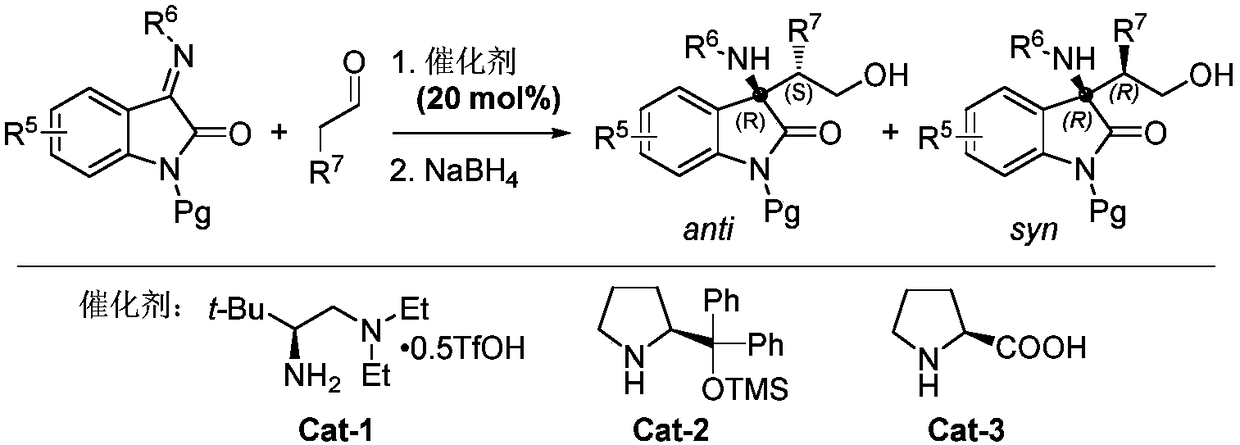
Chiral acyclic secondary amine-tertiary amine catalyst derived from amino acid and preparation method and application thereof
A technology for tertiary amine catalysts and cyclic secondary amines, which is applied in the field of novel chiral acyclic secondary amine-tertiary amine catalysts and its preparation, can solve problems such as catalytic efficiency, stereoselective control or unsatisfactory substrate application range, and achieve The effect of excellent enantioselectivity control ability, excellent non-enantioselectivity control ability, and high-efficiency catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The first specific preparation process of the chiral acyclic secondary amine-tertiary amine catalyst derived from amino acid comprises the following steps:
[0053] Step (1): In a 100mL round-bottomed flask, dissolve N-Boc-protected (S)-tert-leucine (a-1, 2.31g, 10mmol) in 20mL of dry dichloromethane solvent, and then dissolve the It was cooled to 0°C; then, 20 mL of a dry dichloromethane solution of DCC (2.16 g, 10.5 mmol) was slowly added dropwise to the above solution system within 1 h (1 h); during the dropping process, a white solid was observed in the system , after the dropwise addition, the reaction system continued to stir for 30min; within 1h, slowly added 20mL of a dry dichloromethane solution of diethylamine (0.73g, 10mmol) dropwise; after the dropwise addition, the reaction body was transferred The reaction was continued at room temperature for 12 h until the reaction of the raw materials was completed as monitored by TCL. The reaction solution was suction...
Embodiment 2
[0063] The difference between Example 2 and Example 1 is that the amino acid used in step (1) is (S)-phenylglycine protected by N-Boc, the reduction reaction temperature is 0°C, and the LiAlH used in step (2) 4 The amount is 16.2mmol, and other preparation steps and conditions are the same as in Example 1, and 1.45g of chiral secondary-tertiary amine compound of colorless oily liquid is finally obtained, with a total yield of 70%;
[0064] Its structural formula is: The absolute configuration is: S.
[0065] Data characterization of chiral secondary-tertiary amine compound 2: [α] D 20 =+26.1(c 1.5, CHCl 3 ).
[0066] 1 H NMR (400MHz, CDCl 3 ):δ7.37–7.30(m,4H),7.26–7.22(m,1H),3.53(dd,J=10.8Hz,J=3.6Hz,1H),2.70–2.61(m,2H),2.53– 2.41 (m, 5H), 2.29 (s, 3H), 1.02 (t, J=7.2Hz, 6H).
[0067] 13 C NMR (100MHz, CDCl 3): δ142.9, 128.3, 127.5, 127.0, 63.7, 61.3, 47.3, 34.8, 12.0.
[0068] HMRS(ESI): Theoretical [M+H] + C 13 h 23 N 2 is 207.1856, giving m / z 207.1856.
Embodiment 3
[0070] The difference between Example 3 and Example 1 is that the amino acid used in step (1) is N-Boc protected L-phenylalanine, the reduction reaction temperature is 50°C, and the LiAlH used in step (2) 4 The amount is 2.99g, 81mmol, and other preparation steps and conditions are the same as in Example 1, and 0.83g of the chiral secondary-tertiary amine product of a colorless oily liquid is finally obtained, with a total yield of 75%;
[0071] Its structural formula is: The absolute configuration is: S.
[0072] Data characterization of chiral acyclic secondary-tertiary amine compound 3: [α] D 20 =+80.8(c 1.2, CHCl 3 ).
[0073] 1 H NMR (400MHz, CDCl 3 ):δ7.27–7.25(m,2H),7.20–7.16(m,3H),2.85(dd,J=13.6Hz,J=5.2Hz,1H),2.72–2.66(m,1H),2.56– 2.45 (m, 4H), 2.43 (s, 3H), 2.40–2.34 (m, 2H), 2.32–2.25 (m, 2H), 0.94 (t, J=7.2Hz, 6H).
[0074] 13 C NMR (100MHz, CDCl 3 ): δ142.9, 128.3, 127.5, 127.0, 59.7, 57.1, 47.5, 39.2, 34.6, 12.0.
[0075] HMRS(ESI): Theoretical [M+H] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com