Protein carrier for protein transduction and preparation method and application thereof
A protein and carrier technology, applied in the field of cell biology, can solve the problems of toxicological effects, unexplained, safety hazards, etc., and achieve the effect of simple experimental operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Construction of embodiment 1 plasmid vector pEX-4T-MBP-EGFP
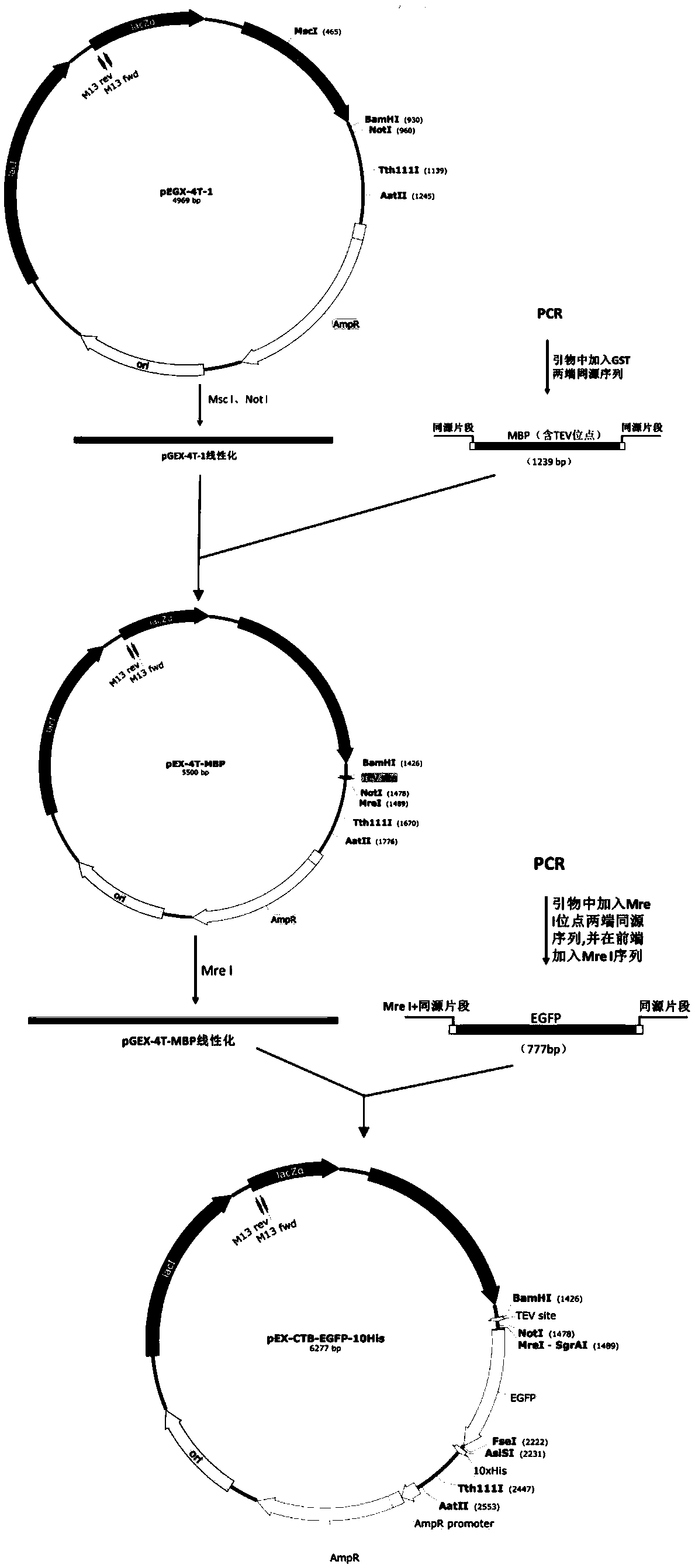
[0061] Use the PCR method to obtain MBP and EGFP gene fragments respectively from wild-type plasmids containing MBP and EGFP, and first clone the MBP gene fragment (amino acid sequence such as SEQ ID NO.2, DNA sequence such as SEQ ID NO.5) into pGEX-4T -1 vector, replace the original GST tag in the vector, and then insert multiple restriction sites to obtain the recombinant plasmid pEX-4T-MBP, and then insert the EGFP gene fragment (amino acid sequence such as SEQ ID NO.4, DNA After the sequence such as SEQ ID NO.7) is inserted into the Mre I restriction site after MBP, the recombinant plasmid pEX-4T-MBP-EGFP is obtained, and its construction diagram is shown in figure 1 As shown, the specific experimental steps are as follows:
[0062] (1) MBP replaces GST label:
[0063] Table 1 The amount of PCR reagents used for MBP gene fragments
[0064]
[0065] The amount of reagents used is shown in Table 1. Fi...
Embodiment 2
[0082] Construction of embodiment 2 plasmid vector pEX-4T-MBP-CTB-EGFP
[0083] The construction flow chart of pEX-4T-MBP-CTB-EGFP is as follows Figure 4 shown. The amount of reagents is shown in Table 5. Search the CTB gene sequence (amino acid sequence such as SEQ ID NO.3, DNA sequence such as SEQ ID NO.6) through NCBI, optimize the sequence to make it suitable for expression in Escherichia coli, and insert it at the front and rear ends Homologous fragment (including restriction enzyme site) of the cloning site (NotI site) in the 20bp destination vector (pEX-4T-MBP-EGFP), synthesized by General Biosystems (Anhui) Co., Ltd. Homologous fragment 1 -CTB-homologous segment 2 gene. Using primers:
[0084] Forward: 5'—ACAAGGACGACGATGACAAG—3', SEQ ID NO.12;
[0085] Reverse: 5'—TGGTGATGATGATGATGATGATG—3', SEQ ID NO.13;
[0086] The homologous fragment 1-CTB-homologous fragment 2 gene was amplified by PCR, the amplification conditions are shown in Table 6, using a high-fidelity...
Embodiment 3
[0092] Embodiment 3 recombinant protein is expressed in Escherichia coli
[0093] (1) Obtain engineering bacteria
[0094] Transform the recombinant plasmid pEX-4T-MBP-CTB-EGFP obtained in Example 2 into Escherichia coli BL21, spread it on LB agar (containing 1‰Amp), pick a single colony after culturing at 37°C for 16 hours, and inoculate In 5mL LB broth (containing 1‰Amp) at 37°C, 200r / min, cultivate for 16h to obtain engineering bacteria.
[0095] (2) induction
[0096] Inject the engineered bacteria obtained in 1 into 300mL LB broth (containing 1‰Amp) at a ratio of 1:100, and cultivate to OD at 37°C and 200r / min 600 When it is 0.6-1.0 (about 3h), add IPTG (final concentration is 0.1mmol / L), immediately place it at 16°C, 200r / min, and cultivate for 16-24h. 4000r / min, 20min, centrifuge at room temperature to collect the bacterial cells, and weigh.
[0097] (3) Enzymatic hydrolysis combined with ultrasonic crushing
[0098] According to the wet weight of the bacteria: enz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com