Application of new cryptolepine derivatives in controlling and preventing plant-derived germs
A technology of selemenine and derivatives, applied in the field of pesticides, can solve problems such as reduced use efficiency, and achieve the effects of high bactericidal activity, excellent bacteriostatic effect and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
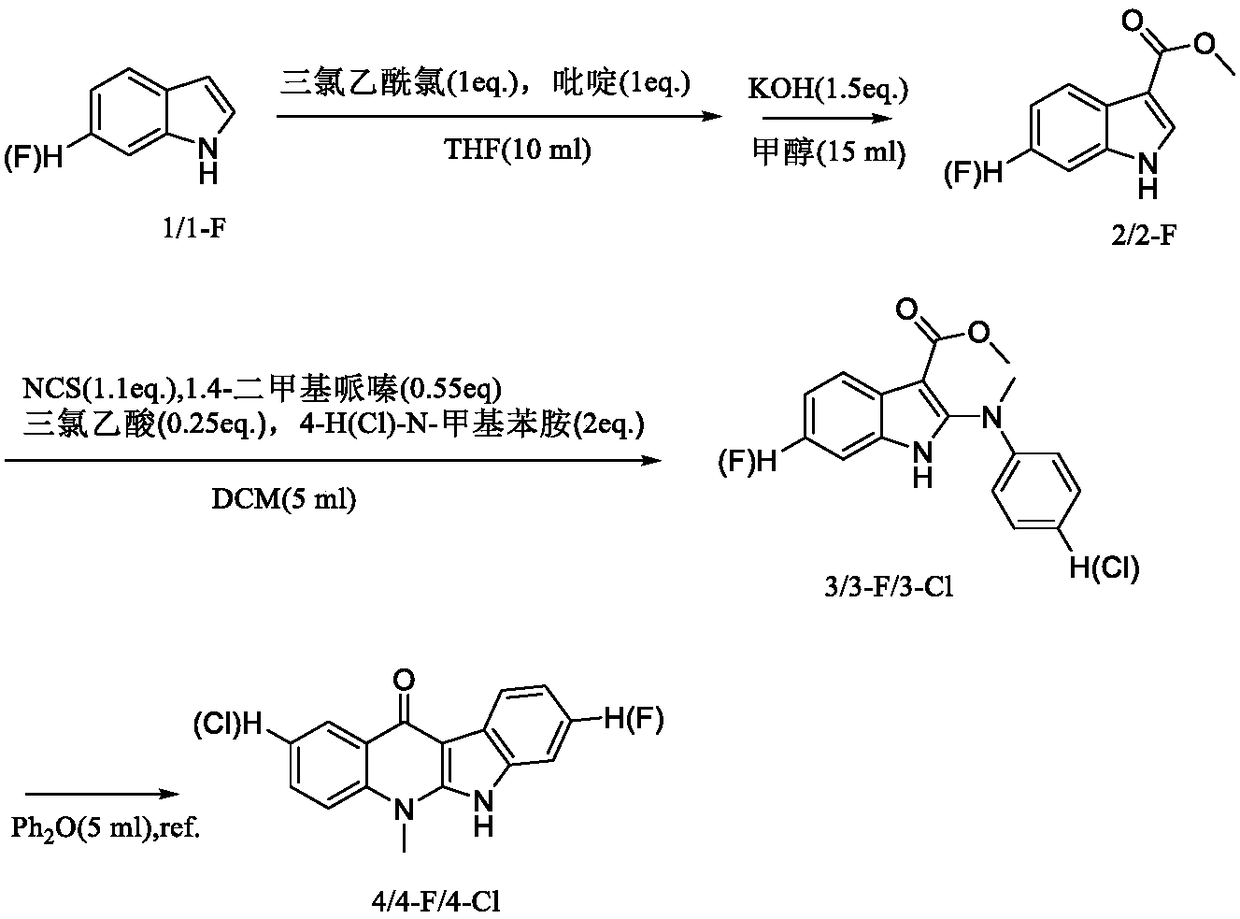
[0015] Synthetic method of intermediate
[0016]
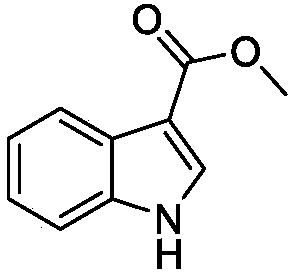
[0017] The synthesis method of intermediates can be found in literature: (1) Lu, W.J., Kathryn, J.W., Wang Li, Kento Imai, et al. European Journal of Medicinal Chemistry, 2013, 64:498-511. (2) Ibrahim, E.S., Pieter , V.D.V., Steert, K., et al.Journal Medical Chemistry, 2009,52:2979-2988. Its specific synthetic operation is described in detail as follows:
[0018] The synthetic method of intermediate 2: pyridine (0.42mL) was added in the tetrahydrofuran (THF) (10mL) solution of indole (0.77mmol) under the condition of ice-bath, then the THF (5mL) solution of trichloroacetyl chloride (0.58mL) ) solution was slowly added dropwise into the above solution with a constant pressure funnel. The mixture was stirred at 25-30°C for 12h. After the completion of the reaction monitored by TLC, the reaction was quenched with 1M HCl (100mL) aqueous solution, extracted three times with ethyl acetate (3×100mL), the organic phase was combin...
Embodiment 2
[0043] The synthetic method of target compound 1a-3a
[0044]
[0045] For the synthesis method of the target compound 1a-3a, please refer to the literature: Ibrahim, E.S., Pieter, V.D.V., Steert, K., et al.. Journal Medical Chemistry, 2009, 52:2979-2988. The specific synthesis operation is described in detail as follows:
[0046] Put the above intermediate 4 (4-F / 4-Cl) (2.0mmol) in a round bottom flask, dissolve it with toluene (5mL), and add POCl after the dissolution is complete 3 (25.0mmol), refluxed at 110°C for 12h, after TLC monitored the reaction was complete, cooled to 25°C, spin-dried the toluene solution, washed with ice saturated NaHCO 3 Neutralize the product with an aqueous solution (maintain a neutral environment at 30°C), extract the organic phase with dichloromethane as the reaction solution, wash the organic phase with a saturated NaCl aqueous solution, combine the organic phases and wash with anhydrous NaCl 2 SO 4 After drying, the organic phase was spi...
Embodiment 3
[0057] The synthetic method of target compound 4a-24a
[0058]
[0059]
[0060] For the synthesis method of the target compound 4a-24a, please refer to the literature method: Mei, Z.W., Wang, L., Lu, W.J., etal. Journal Medical Chemistry, 2013, 56:1431-1442. The specific synthesis operation is described in detail as follows:
[0061] Put the above target compound 1a (2a / 3a) (1.0mmol) and the corresponding aminoalkylamine reagent (10.0mmol) in a round bottom flask, dissolve it with DMF (10mL), and wait until the temperature rises to 135-155°C It was refluxed for 1-4 hours, and after the completion of the reaction as monitored by TLC, the DMF was spin-dried under reduced pressure. The resulting residue was purified by silica gel column chromatography using ethyl acetate and methanol containing 2N ammonia as eluents.
[0062] Target compound 4a:
[0063]
[0064] Yellow solid; Yield: 84%; 1 H NMR (400MHz, CDCl 3 )δ8.47(1H,d,J=8.0Hz,Ar-H),8.28(1H,d,J=8.0Hz,Ar-H),7.68...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com