Method for inducing and differentiating amniotic epithelial stem cells into functional liver cells and application of method
A technology for inducing differentiation and stem cells, applied in cell dissociation methods, cell culture active agents, biochemical equipment and methods, etc., can solve the problems of stem cell tumorigenicity, limited sources of bone marrow and umbilical cord blood, and difficult clinical use of cells, etc. Achieve the effects of inhibiting transaminase activity, restoring liver albumin levels, and enriching cell sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
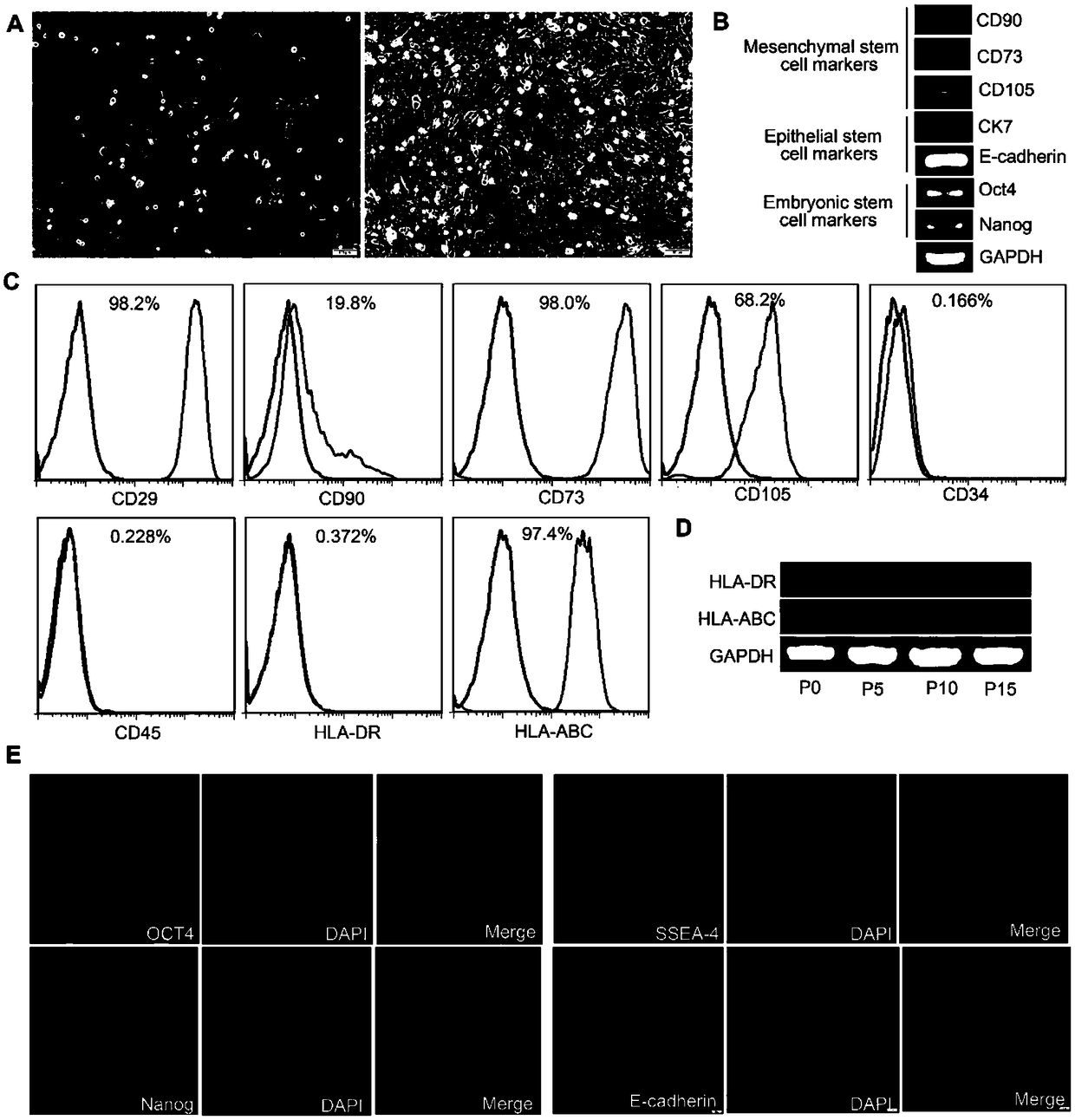
[0089] Example 1. Isolation, culture, expansion and GFP marker of amniotic membrane epithelial stem cells (HAESCs):
[0090] Under the premise of obtaining the consent of the newborn's family members, the fresh amniotic membrane was isolated. After 2 hours of antibiotic treatment, microbiological detection and safety detection of infectious disease pathogens were carried out. After the amniotic membrane was digested by trypsin / EDTA (0.25%) in a water bath at 37°C for 1 hour, stop solution was added. After centrifugation, the supernatant was removed, and the amnion epithelial stem cell culture medium was placed in 5% CO 2 , 95% humidity, and cultured in a 37°C incubator. Change the medium after 2-3 days to remove unattached cells, and then change the medium every 2 days. When the confluence of the cells reached 80%, trypsin / EDTA was used for passage. The amniotic epithelial stem cells of passage 3 were transfected with GFP using lentivirus. Immunofluorescence and flow cyto...
Embodiment 2
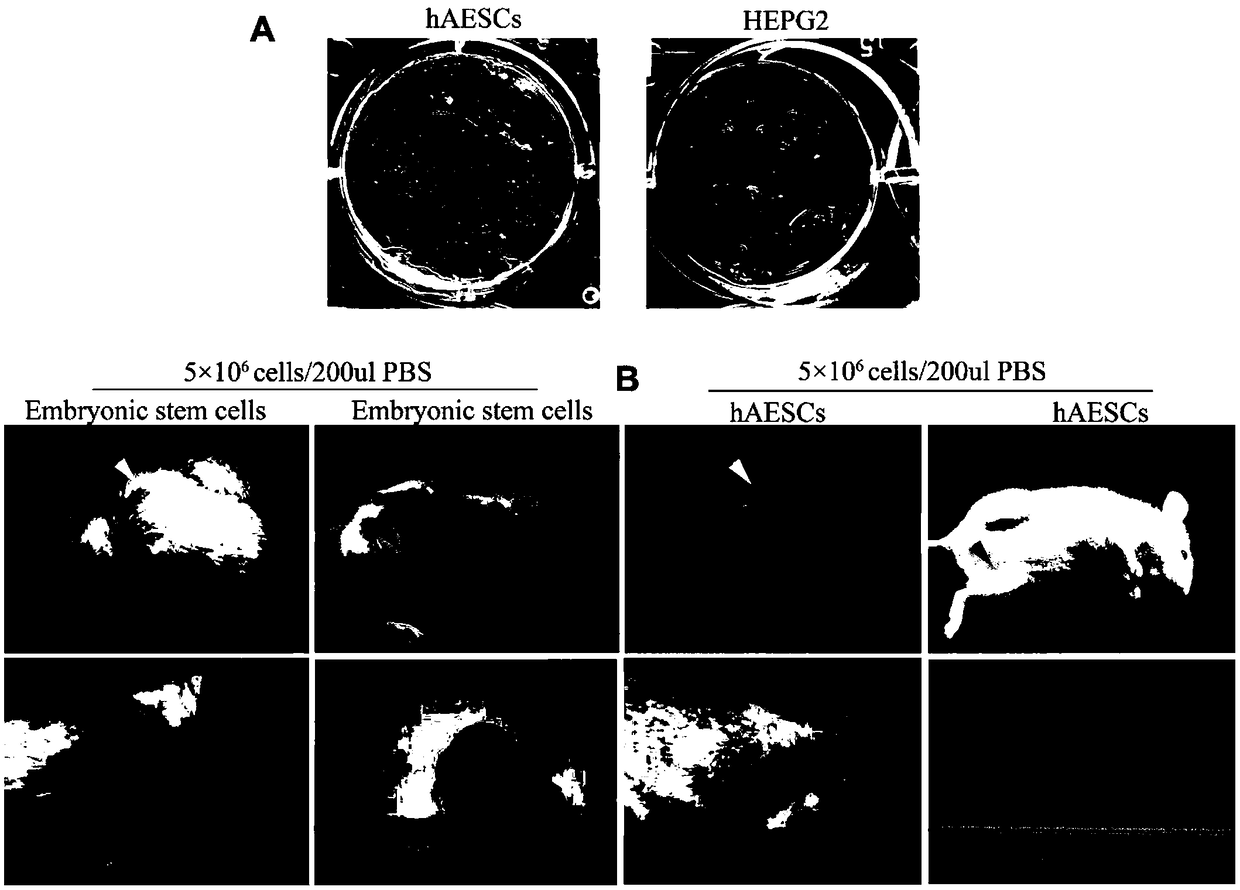
[0176] Example 2. In vivo and in vitro tumorigenicity detection of amniotic membrane epithelial stem cells
[0177] 1. In vivo and in vitro tumorigenicity detection of amniotic membrane epithelial stem cells
[0178]1) Take the amniotic membrane epithelial cells with good growth condition in the third generation of culture, showing vigorous growth, large cell bodies, clear nuclei, abundant cytoplasm, and strong refraction under the microscope. ) digestion, when the cells turned into a single circle under the microscope, the digestion was terminated with DMEM medium containing 10% (volume concentration) fetal bovine serum, gently pipetted and centrifuged to obtain cell pellets, washed with PBS (PBS without calcium and magnesium ions). (the purpose is to wash off trypsin, fetal bovine serum and other substances);
[0179] 2) the amniotic membrane epithelial stem cells in step 1) were inoculated on soft agar, and after culturing for 30 days, the colony formation was observed;
...
Embodiment 3
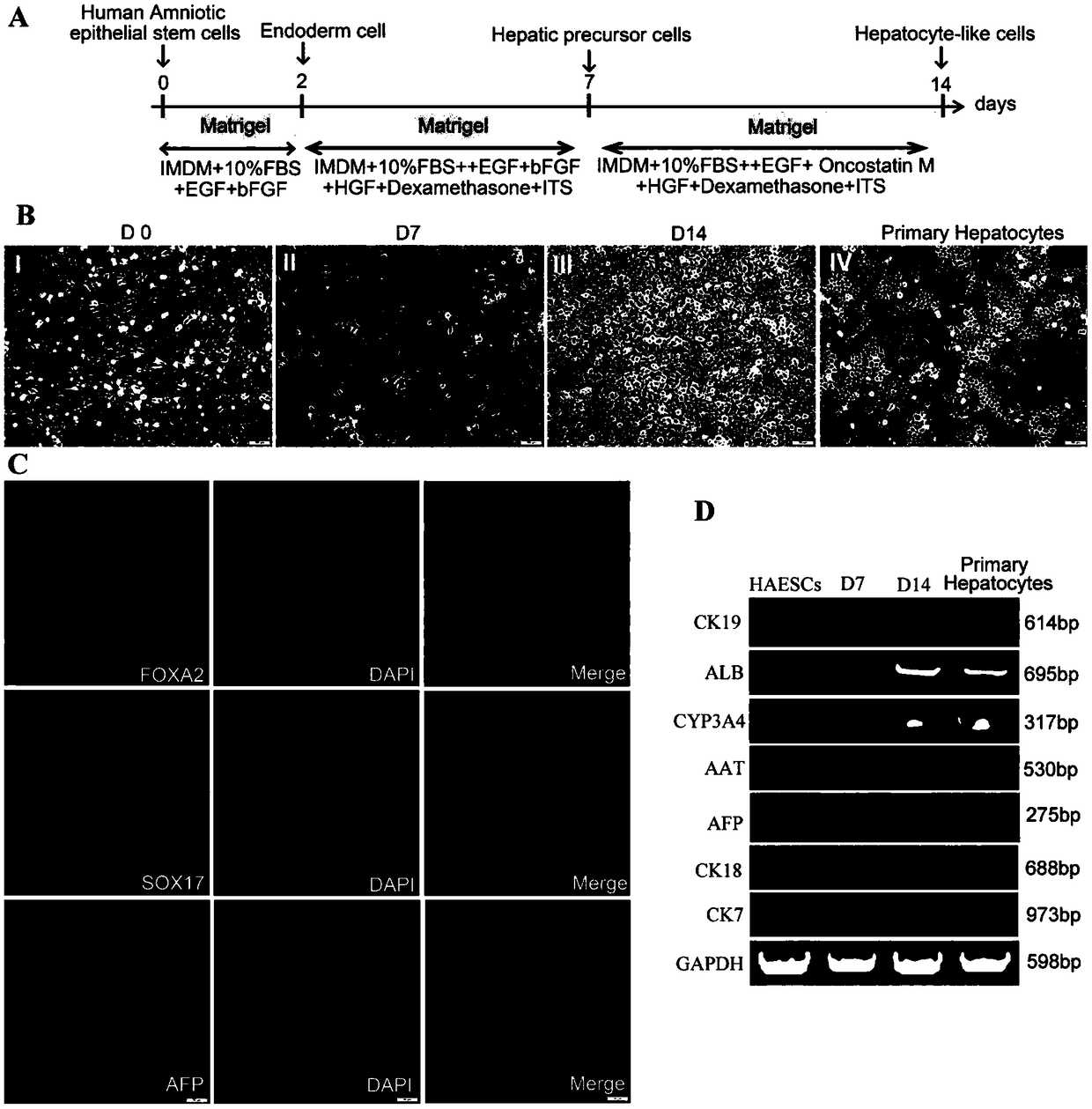
[0187] Example 3. Induction and differentiation of amniotic epithelial stem cells into hepatocytes and their functional detection
[0188] The specific operation procedures are as follows:
[0189] 1. In vitro directional induction of amniotic epithelial stem cells to differentiate into hepatocytes
[0190] 1) Take the well-grown cells of the third generation of culture and digest them with trypsin-EDTA digestion solution (0.25%). DMEM medium to terminate the digestion, gently pipetting and centrifugation to obtain cell pellets, washed twice with PBS (PBS washing solution without calcium and magnesium ions) (the purpose is to wash away substances such as trypsin and fetal bovine serum);
[0191] 2) The above-mentioned cell pellets washed with PBS were inoculated into a 6-well plate pretreated with Matrigel for induction culture, and the inoculated cell density was 3 × 10 5 1 / well, add 2ml of amniotic epithelial stem cell universal medium to each well, at 37°C, 5% CO 2 , cul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com