A2-(pi-A1)2 wide band gap non-fullerene receptor material based on diphenylthiophenyl sulfone and preparation method and application thereof
A technology of diphenylthiophenylsulfone and gap non-fullerene is applied in the field of A22 type wide band gap non-fullerene acceptor material and its preparation, which can solve the problem of complex preparation process and few varieties of wide band gap small molecule acceptor materials. and other problems, to achieve narrow spectral absorption, promote π-π stacking, and promote the transmission and separation of excitons.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] A 2 (π-A 1 ) 2 Synthesis of intermediates of wide bandgap small molecule non-fullerene acceptor materials (M1, M2, M3, M4, M5, M6 and M7), the synthesis route is shown below.
[0069]
[0070] 1.1 Synthesis of 2-(tri-n-butyltin base)-4-butyloctylthiophene (M1)
[0071]In a 100mL three-necked flask, add 2-(2-butyloctyl)thiophene (3.0g, 11.9mmol) and 40mL of freshly distilled tetrahydrofuran, stir magnetically under nitrogen protection, place the system at -78°C, and automatically Slowly add n-butyl lithium (8.5mL, 1.6M) dropwise into the constant pressure dropping funnel, after the dropwise addition, continue the reaction at low temperature for 2h, then add tributyltin chloride (4.60g, 14.3mmol) dropwise, and continue the reaction After 30min, move to room temperature for 24h. After the reaction was completed, the reaction solution was poured into 150 mL of water to quench the reaction, and the mixed solution was extracted with dichloromethane (50 mL × 3 times), t...
Embodiment 2
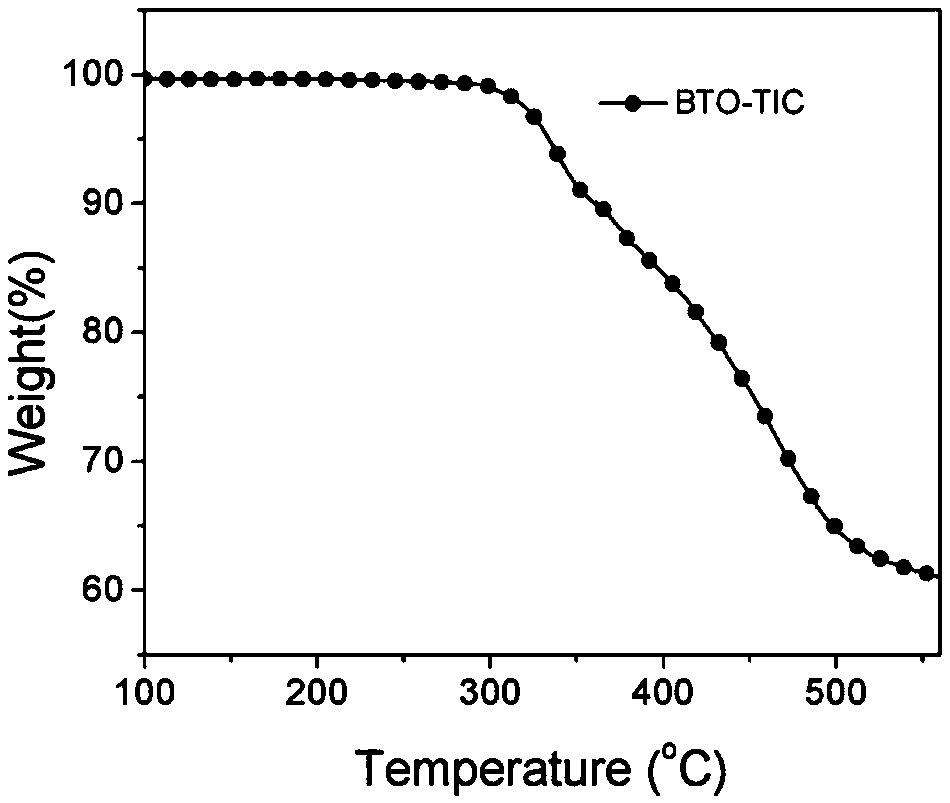
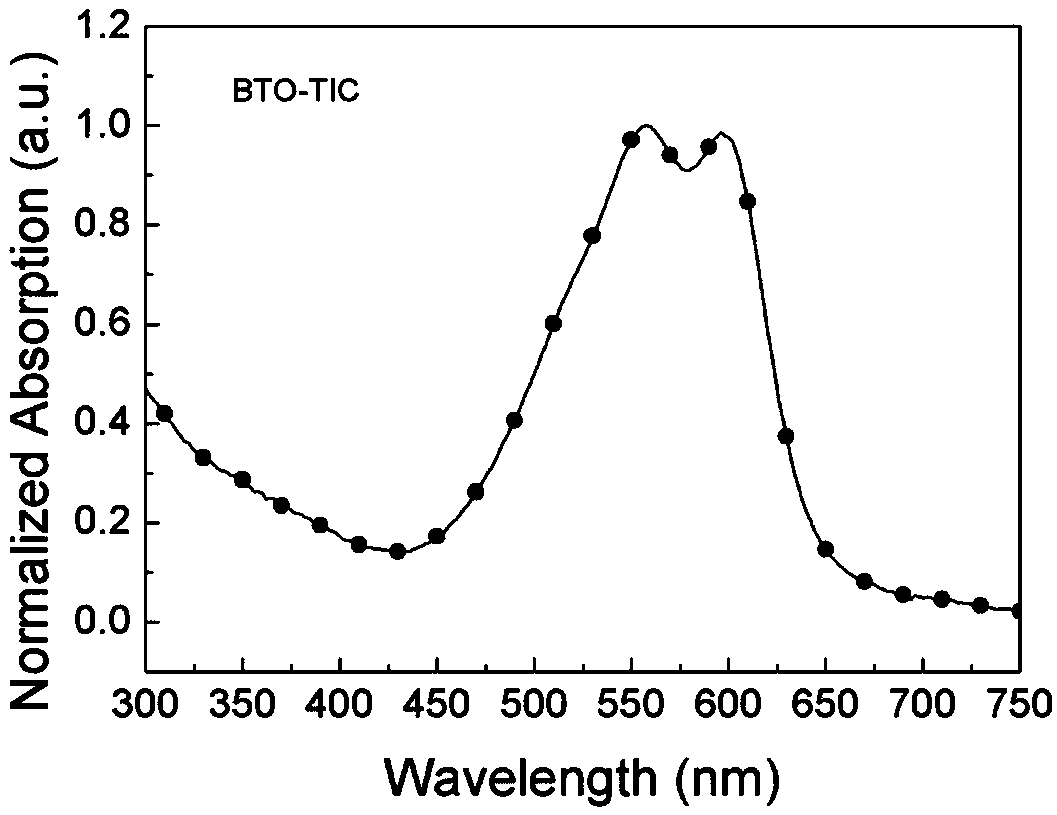
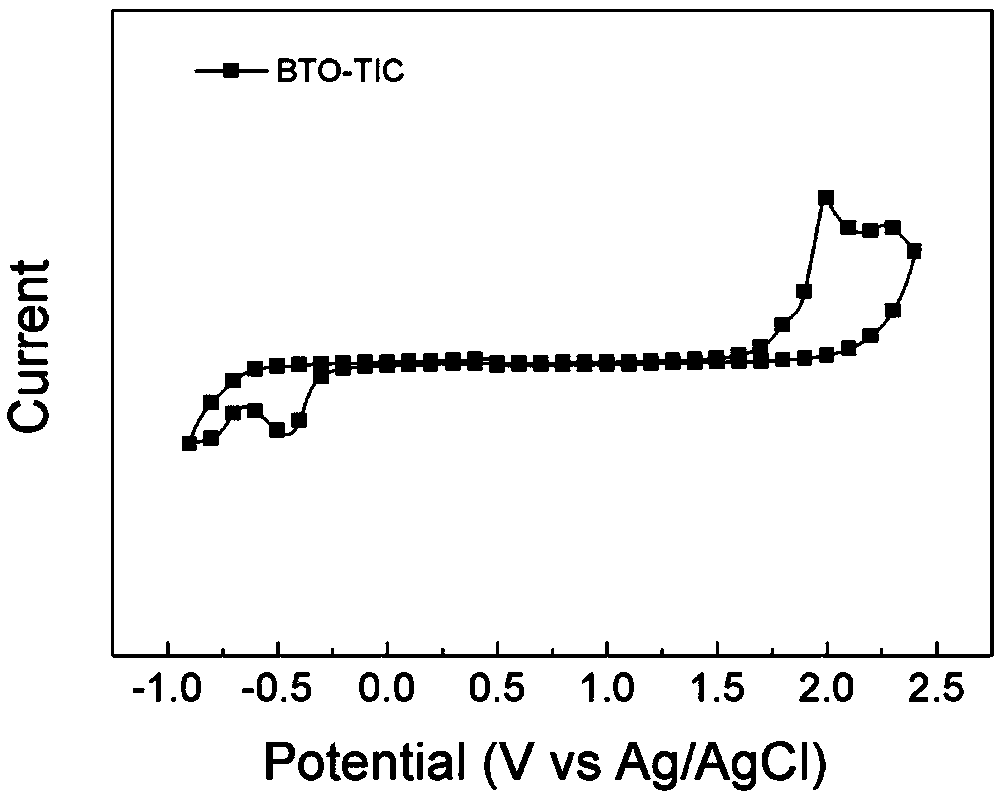
[0084] Example 2 Synthesis of wide bandgap non-fullerene acceptor BTO-TIC
[0085]
[0086] Add compound 7 (100mg, 0.14mmol), 3-(dicyanomethylene) indanone (130mg, 0.67mmol) and 15mL of freshly distilled tetrahydrofuran to a 50mL single-necked bottle in turn, stir magnetically, and the solid in the system dissolves completely Finally, 5 drops of pyridine were added dropwise, the system reacted rapidly, and reacted for 12 hours at room temperature. After the reaction, the reaction solution was poured into 150mL water to quench the reaction, and the mixed solution was extracted with chloroform (50mL×3 times), the organic phases were combined, anhydrous MgSO 4 Dry, filter, and spin off the solvent to obtain a purple solid crude product. The crude product is separated by column chromatography using dichloromethane / ethyl acetate=40 / 1 as the eluent to obtain a purple solid (100mg, yield=62% ). 1 H NMR (400MHz, CDCl 3 )δ8.99 (s, 2H), 8.71 (d, J = 7.1Hz, 2H), 8.20 (s, 2H), 8.02 ...
Embodiment 3
[0087] Example 3 Synthesis of wide bandgap non-fullerene acceptor BTO-TDFIC
[0088]
[0089] Refer to the synthesis and purification method of the target compound BTO-TIC. The eluent was dichloromethane / ethyl acetate=40 / 1 to obtain a purple solid (120 mg, yield=66%). 1 H NMR (400MHz, CDCl 3 )δ9.02(s,2H),8.58(dd,J=9.8,6.5Hz,2H),8.23(s,2H),8.08(d,J=8.0Hz,2H),7.90(d,J=8.1 Hz,2H),7.73(s,2H),7.48(s,2H),2.94(d,J=7.3Hz,4H),1.77(s,2H),1.30-1.25(m,32H),0.90-0.84 (m,12H).Anal.Calcd for:C 70 h 64 f 4 N 4 o 4 S 3 : C, 70.21; H, 5.39; N, 4.68; S, 8.03. Found: C, 68.54; H, 5.30; N, 4.72; S, 8.08.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com