Method for efficiently synthesizing optically active poly-substituted 2,3-dihydrobenzofuran compound through catalysis of asymmetric organic phosphine
A compound, the technology of dihydrobenzene, applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems rarely reported, such as Yang Qingqing et al., and achieve good industrial application prospects, low cost, and small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
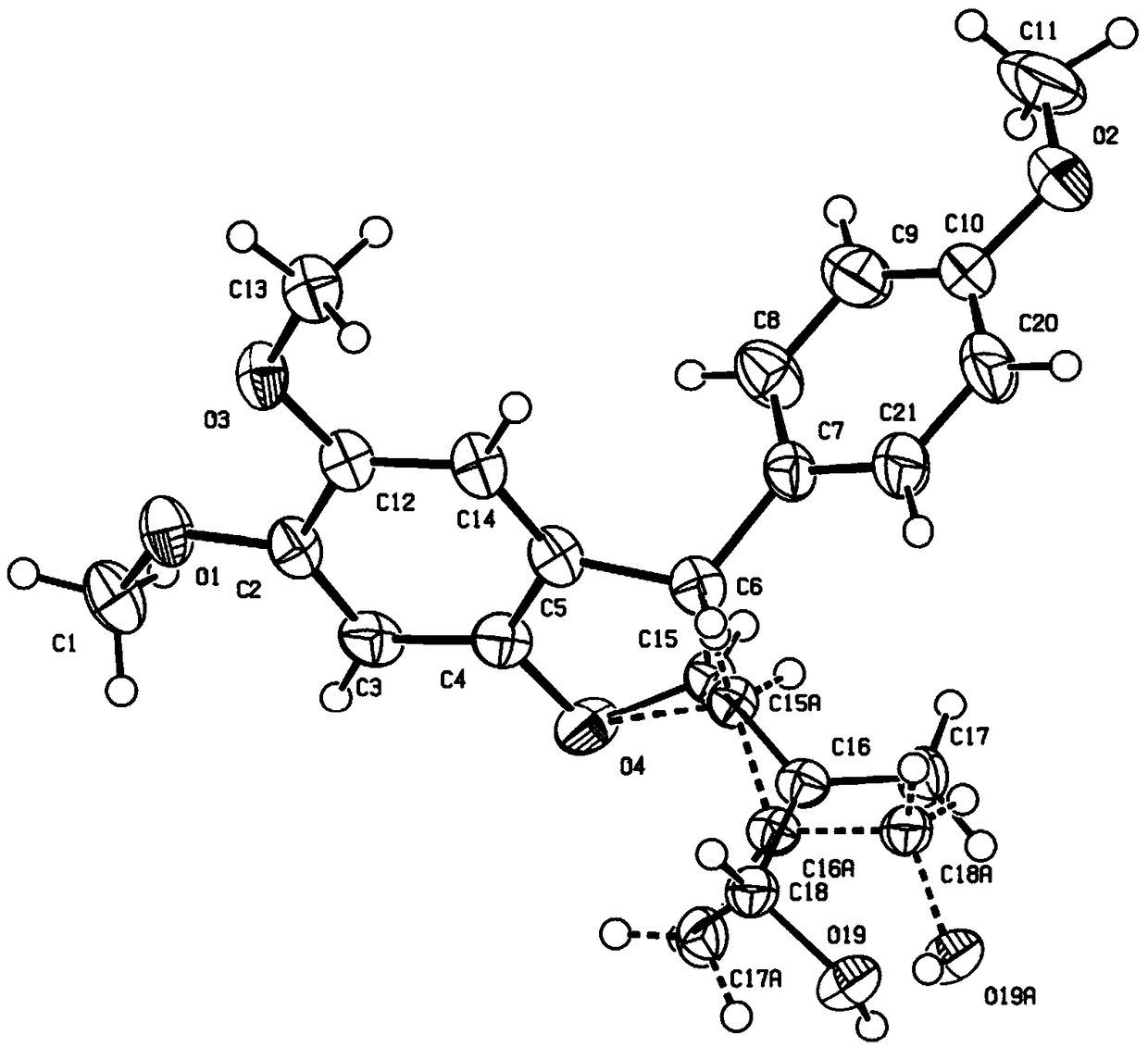
[0032] In a sealed tube under nitrogen atmosphere, a mixture of compound la (0.2 mmol), compound 2a (0.3 mmol), catalyst I (0.02 mmol) in MeCN (2.0 mL) was stirred at 25 °C for 4.5 h. After removal of the solvent, the crude residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate) to give the corresponding product 3aa.
[0033]
[0034] Yield 70%, dr>20:1, 73% ee.
[0035] 1 H NMR (500Hz, CDCl 3 ): δ (ppm) 7.10 (d, J = 8.5Hz, 2H), 6.85 (d, J = 9.0Hz, 2H), 6.51 (s, 1H), 6.43 (s, 1H), 6.30 (s, 1H) ,5.89-5.87(m,3H),5.31(d,J=5.0Hz,1H),4.30(d,J=5.0Hz,1H),3.78(s,3H),3.74(s,3H). 13 C NMR (126Hz, CDCl 3 ): δ (ppm) 166.0, 158.7, 154.0, 147.9, 142.3, 139.3, 135.5, 128.8, 125.5, 120.8, 114.1, 105.6, 101.4, 93.0, 89.6, 55.4, 54.4, 52.0. HRMS: Accurate mass calculation [M+ H] + (C 20 h 19 o 6 ) is m / z 355.11761, and the measured value is m / z 355.11679.HPLC conditions: Daicel Chiralpak OD-H column, n-hexane / isopropanol=90 / 10, flow rate=1.0mL / min, λ...
Embodiment 2
[0037] In a sealed tube under nitrogen atmosphere, a mixture of compound la (0.2 mmol), compound 2a (0.3 mmol), catalyst II (0.02 mmol) in MeCN (2.0 mL) was stirred at 25 °C for 4.5 h. After removal of the solvent, the crude residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate) to give the corresponding product 3aa.
[0038] Yield 60%, dr>20:1, -63% ee.
[0039]
Embodiment 3
[0041] In a sealed tube under nitrogen atmosphere, a mixture of compound la (0.2 mmol), compound 2a (0.3 mmol), catalyst III (0.02 mmol) in MeCN (2.0 mL) was stirred at 25 °C for 4.5 h. After removal of the solvent, the crude residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate) to give the corresponding product 3aa.
[0042] Yield 66%, dr>20:1, 80% ee.
[0043]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com