Irinotecan hydrochloride-containing drug composition and preparation method thereof
A technology for irinotecan hydrochloride and its composition, which is applied in the field of pharmaceutical composition containing irinotecan hydrochloride and its preparation, can solve the problems of failure to meet the registration standards of imported drugs, poor stability of original research products, and high equipment requirements, etc., and achieve reduction The effect of drug risk, no special equipment requirements, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085]
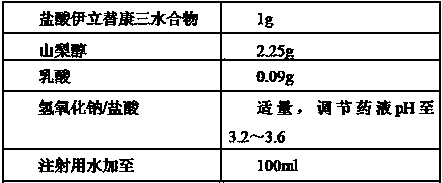
[0086] Step (1): Pre-dissolve lactic acid with water for injection at 65°C~75°C and add it to the mixing tank;
[0087] Step (2): Pre-dissolve irinotecan hydrochloride with water for injection at 20°C to 30°C and add it to the mixing tank;
[0088] Step (3): Pre-dissolve sorbitol with water for injection at 20°C to 30°C and add it to the mixing tank;
[0089] Step (4): Pass process hot water through the jacket of the liquid mixing tank to heat the liquid medicine to 65℃~75℃;
[0090] Step (5): Add water for injection to 80%-85% of the prepared volume, mix well, pass chilled water through the mixing tank to control the temperature of the liquid to 20℃~30℃, check that the solution should be a light yellow clear liquid;
[0091] Step (6): Adjust the pH of the drug solution to 3.2 with sodium hydroxide solution or hydrochloric acid solution, and then add water for injection at 20°C to 30°C to the final volume;
[0092] Step (7): Fill the batching tank with nitrogen filtered by a 0.2...
Embodiment 2
[0100]
[0101] Prepare injection according to the following method:
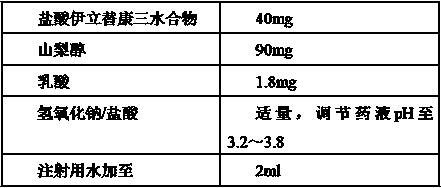
[0102] Step (1): Pre-dissolve lactic acid with water for injection at 65°C~75°C and add it to the mixing tank, adjust the pH to 4.0 with sodium hydroxide solution or hydrochloric acid solution;
[0103] Step (2): Pre-dissolve irinotecan hydrochloride with water for injection at 20°C to 30°C and add it to the mixing tank;
[0104] Step (3): Pre-dissolve sorbitol with water for injection at 20°C to 30°C and add it to the mixing tank;
[0105] Step (4): Heat the liquid medicine to 65℃~75℃ through process hot water in the jacket of the liquid mixing tank, and stir to dissolve the medicine;
[0106] Step (5): Add water for injection to 80%-85% of the prepared volume, mix well, pass chilled water through the mixing tank to control the temperature of the liquid to 20℃~30℃, check that the solution should be a light yellow clear liquid;
[0107] Step (6): Adjust the pH of the drug solution to 3.2 with sodium hydroxide solution...
Embodiment 3
[0116] Prepare injections according to the prescription described in Example 1 and the preparation method described in Example 1. The difference is that step (1) pre-dissolve lactic acid with water for injection at 65°C~75°C and add it to the dosing tank, then use sodium hydroxide The solution or hydrochloric acid solution adjusts the pH of the lactic acid aqueous solution to 4.5, and then performs subsequent operations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com