Impurities of baricitinib and preparation and detection methods thereof
A baricitinib and impurity technology, which is applied to medical preparations containing active ingredients, measuring devices, and pharmaceutical formulas, can solve problems such as optimization and selection, and achieve the effects of ensuring safety and reliability, simple operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 impurity A
[0051] Weigh baricitinib (1.0g, 2.7mmol) into a 100ml reaction flask, add 20ml DMSO, stir to dissolve, add potassium carbonate (0.6g, 4.3mmol), cool to below 10°C, slowly drop into 4.0ml 30% hydrogen peroxide, naturally warmed up to room temperature after dripping, stirred for about 10 minutes, after TLC confirmed that the reaction was complete, added 100ml of water, extracted with ethyl acetate (30ml×3), combined organic phases, washed with water (30ml×2), saturated saline Washed, dried over anhydrous sodium sulfate for 30 min, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 500 mg of a white solid product with a yield of 47.7% and an HPLC purity of 96.96%.
[0052] MS-ESI(M+1): m / z 391.1;
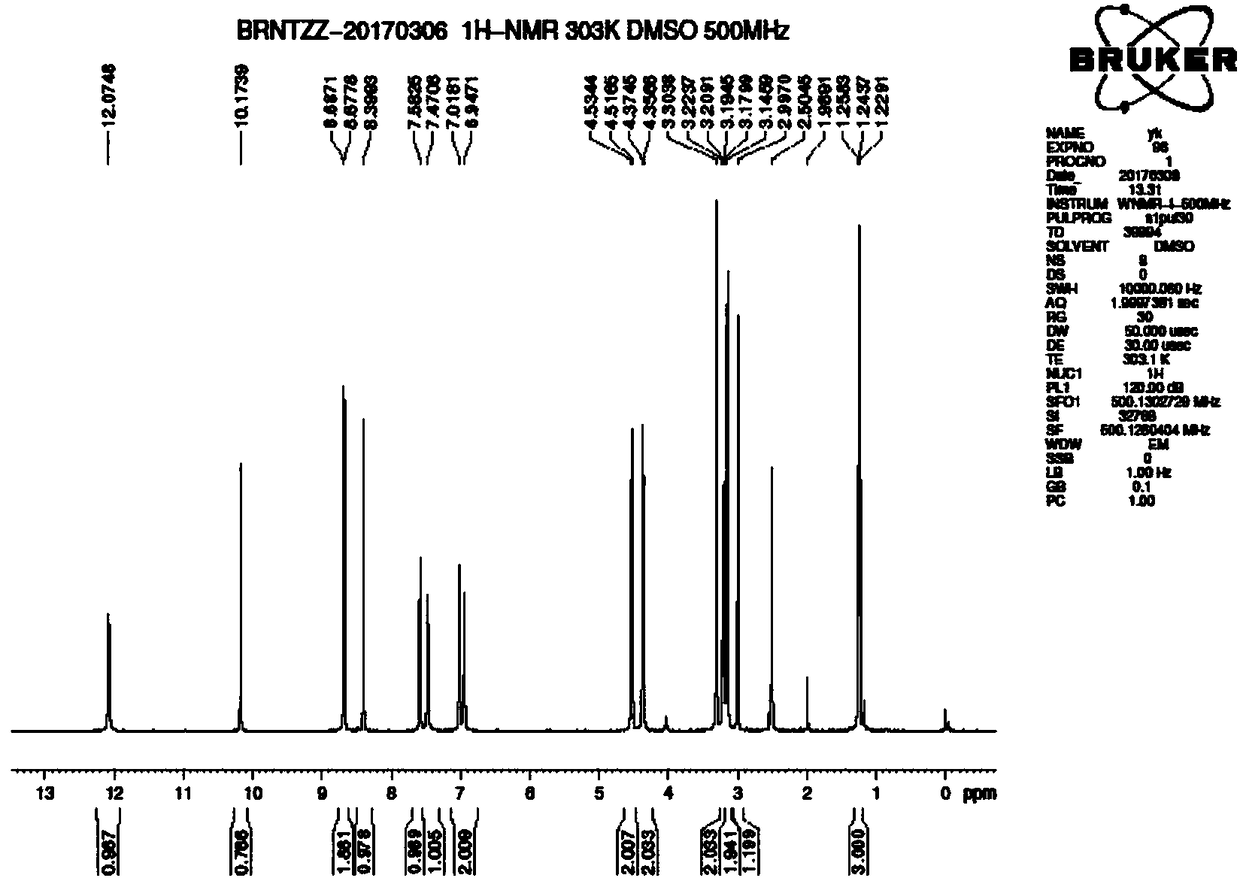
[0053] 1 H NMR (500MHz, d 6 -DMSO)δ:12.07(s,1H),10.17(s,1H),8.68(d,2H),8.40(s,1H),7.58(d,1H),7.47(d,1H),6.98(d ,2H),4.53(d,2H),4.37(d,2H),3.30(s,2H),3.15-3.22(m,2H),3.00(s,2H),1.24(t,3H).
Embodiment 2
[0054] The preparation of embodiment 2 impurity A
[0055]Take baricitinib (1.0g, 2.7mmol) and put it into a 50ml reaction flask, add 15ml methanol and 1ml DMSO, then add 6ml of 1mol / L NaOH solution and 2ml H at room temperature 2 o 2 . The reaction mixture was heated at 50° C. for 3 h. After TLC confirmed that the reaction was complete, it was cooled to room temperature, 100 ml of water was added, extracted with ethyl acetate (30 ml × 3), the organic phases were combined, washed with water (30 ml × 2), and washed with saturated brine. Dry over sodium sulfate for 30 min, filter, and concentrate the filtrate to dryness under reduced pressure to obtain 612 mg of a white solid product with a yield of 58.4% and a purity of 95.98% by HPLC.
Embodiment 3
[0056] Example 3 Preparation of Impurity B
[0057] Weigh baricitinib (2.0g, 5.4mmol) into a 100ml reaction bottle, slowly add 30ml of concentrated hydrochloric acid, heat up to 100°C, react for 1-2h, after TLC confirms that the reaction is complete, add 30ml of water, cool to 0 ℃, use 30% NaOH solution to adjust the pH to about pH9~10, extract with ethyl acetate (50ml×3), combine the organic layers, wash with water (50ml×2), dry over anhydrous sodium sulfate for 30min, filter, and put the filtrate at 45℃ Concentrate to dryness under reduced pressure to obtain 800 mg of off-white solid with a yield of 38.1% and an HPLC purity of 94.67%.
[0058] MS-ESI(M+1): m / z 391.1
[0059] 1 H NMR (300MHz, d 6 -DMSO)δ:13.19(brs,1H),9.20(s,1H),8.94(s,1H),8.75(s,1H),7.96(s,1H),7.61(t,1H),7.44(s ,1H),4.85(d,1H),4.69(d,1H),3.68(s,2H),3.27-3.51(m,2H),2.95(q,2H),1.03-1.15(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com