Method for preparing orthopaedic implant calcium phosphate coating
A calcium phosphate and orthopedic technology, applied in the field of medical biomaterials, can solve the problems of poor universality of electrochemical calcium phosphate coatings, and achieve strong promotion, improved efficiency, and good electrochemical coating effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
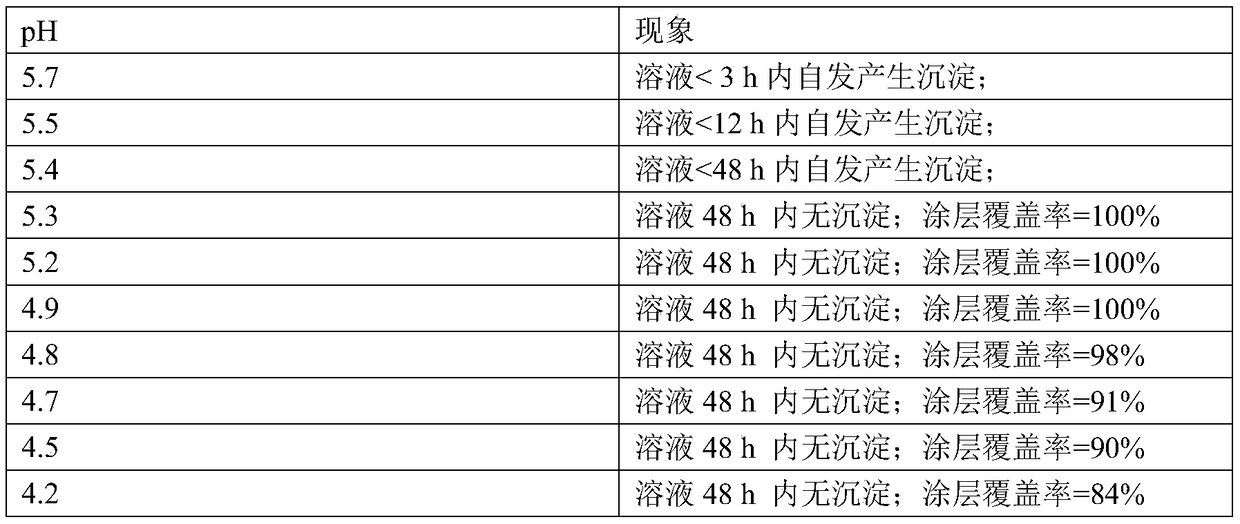
[0085] Determining an electrolyte that satisfies optimum electrolyte conditions in a calcium phosphate electrochemical coating comprises the following steps;
[0086] (a) Calcium nitrate 2mM, ammonium dihydrogen phosphate 4mM; calcium nitrate 4mM, ammonium dihydrogen phosphate 8mM; calcium nitrate 8mM, ammonium dihydrogen phosphate 16mM; calcium nitrate 10mM, ammonium dihydrogen phosphate 20mM; calcium nitrate 15mM, phosphoric acid Ammonium dihydrogen 30mM; calcium nitrate 20mM, ammonium dihydrogen phosphate 40mM; a series of mixed solutions of calcium nitrate 25mM and ammonium dihydrogen phosphate 50mM;
[0087] (b) Titrate each group of mixed solutions prepared in step a with NaOH solution with a concentration of 0.1mM until the solution begins to appear turbidity visible to the naked eye, and the turbidity does not disappear after shaking for 30 seconds. Set this point as the titration End point, and record the volume of the NaOH solution that each group of mixed solutions is...
Embodiment 2
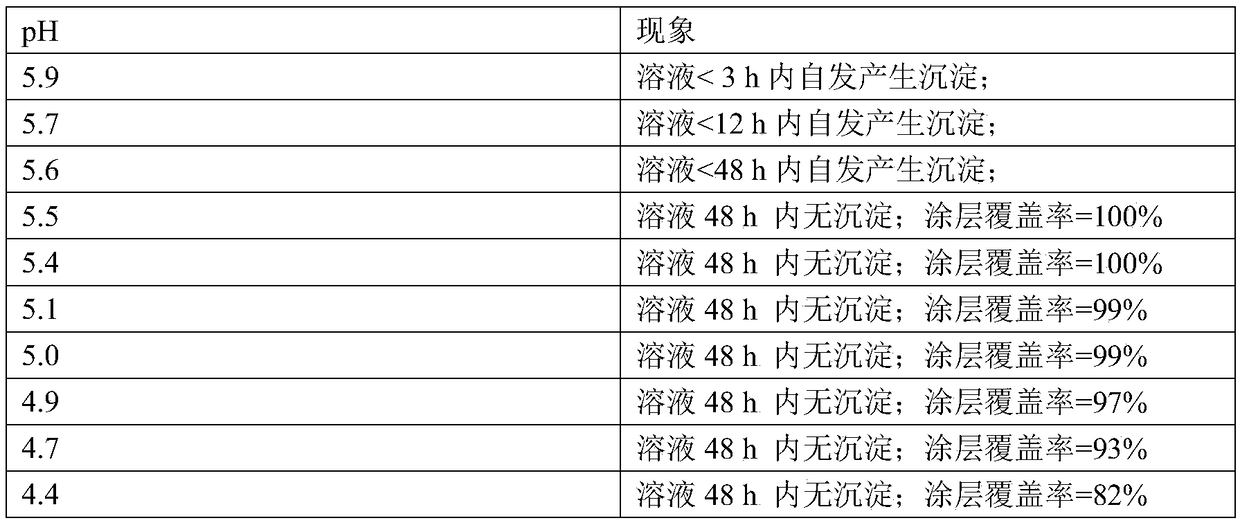
[0098] On the basis of embodiment 1, carry out following steps:
[0099] (a) Calcium nitrate 2mM, ammonium dihydrogen phosphate 1.2mM; calcium nitrate 4mM, ammonium dihydrogen phosphate 2.4mM; calcium nitrate 6mM, ammonium dihydrogen phosphate 3.6mM; calcium nitrate 8mM, ammonium dihydrogen phosphate 4.8mM; Calcium 10mM, ammonium dihydrogen phosphate 6mM; calcium nitrate 15mM, ammonium dihydrogen phosphate 9mM; series of mixed solutions of calcium nitrate 20mM, ammonium dihydrogen phosphate 12mM;
[0100] (b) Titrate each group of mixed solutions prepared in step a with NaOH solution with a concentration of 0.1mM until the solution begins to appear turbidity visible to the naked eye, and the turbidity does not disappear after shaking for 30 seconds. Set this point as the titration End point, and record the volume added dropwise of each group of mixed solutions;
[0101] (c) measure the pH value of each group of mixed solutions at the end of titration, and calculate the concen...
Embodiment 3
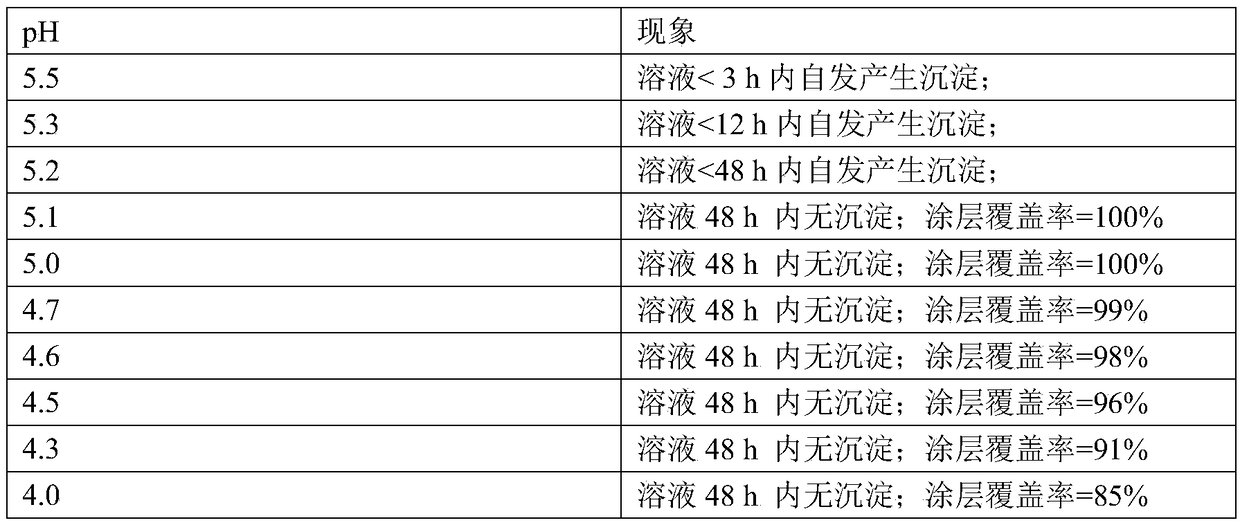
[0111] On the basis of above-mentioned embodiment, carry out following steps:
[0112] (a) Calcium nitrate 2mM, potassium dihydrogen phosphate 1.2mM; calcium nitrate 4mM, potassium dihydrogen phosphate 2.4mM; calcium nitrate 6mM, potassium dihydrogen phosphate 3.6mM; calcium nitrate 8mM, potassium dihydrogen phosphate 4.8mM; nitric acid Calcium 10mM, potassium dihydrogen phosphate 6mM; calcium nitrate 15mM, potassium dihydrogen phosphate 9mM; series of mixed solutions of calcium nitrate 20mM, potassium dihydrogen phosphate 12mM;
[0113] (b) Titrate each group of mixed solutions prepared in step a with NaOH solution with a concentration of 0.1mM until the solution begins to appear turbidity visible to the naked eye, and the turbidity does not disappear after shaking for 30 seconds. Set this point as the titration End point, and record the volume added dropwise of each group of mixed solutions;
[0114] (c) measure the pH value of each group of mixed solutions at the end of ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com