Active amino cross-linked phosphazene derivative and preparation method and application thereof, and flame-retardant epoxy resin
An epoxy resin and cross-linking technology, which is applied in the field of phosphazene derivatives, can solve the problems of poor compatibility of epoxy resin substrates, etc., and achieve good flame retardancy and comprehensive mechanical properties, high char formation, and high limit Effect of Oxygen Index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Add 1.7383g of hexachlorocyclotriphosphazene (HCCP) to 30ml of dioxane, and ultrasonically disperse at room temperature to obtain a uniform solution; take 3.876g of N-aminoethylpiperazine (AEP) and 3.0357g of tris Ethylamine (TEA) was added to a three-necked flask filled with 20ml of dioxane; the molar ratio of HCCP and AEP was controlled to be 1:6. The solution of hexachlorocyclotriphosphazene was dropped into a three-necked flask filled with triethylamine and N-aminoethylpiperazine using a constant pressure dropping funnel, and reacted ultrasonically at 40°C for 3.5h. After the reaction is finished, after cooling to room temperature, the product is centrifuged, washed with dioxane and deionized water three times in sequence, and the obtained solid product is placed in a blast drying oven at a drying temperature of 120°C. After drying for 12 hours, an active amino cross-linked phosphazene derivative was obtained.
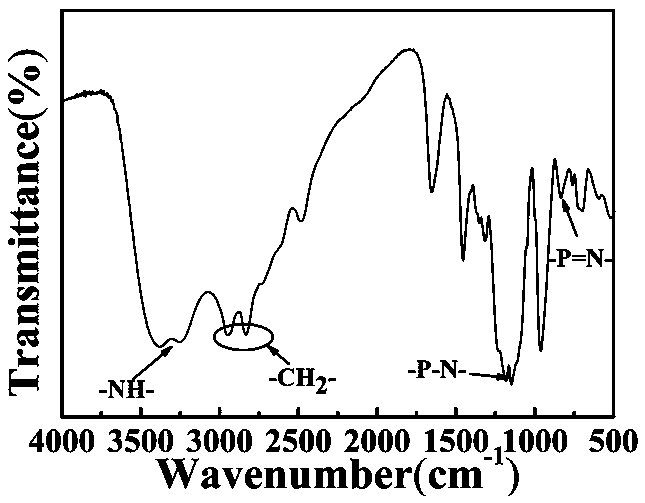
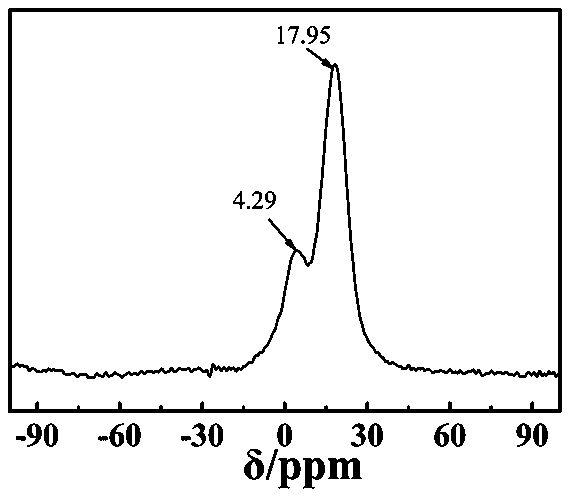
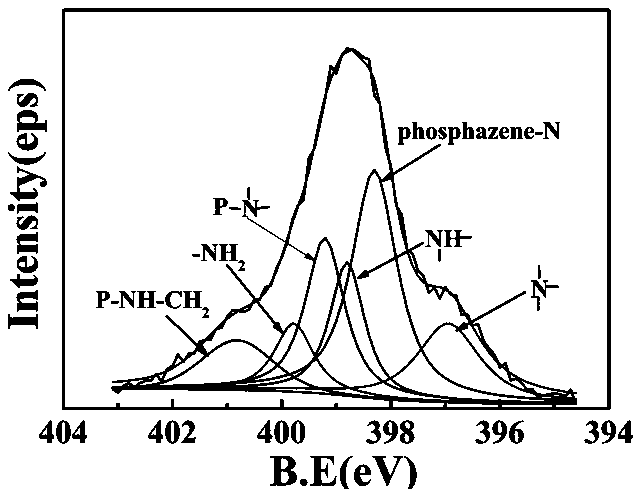
[0049] Infrared, NMR, and X-ray photoelectron spectro...
Embodiment 2
[0054] Add 1.8g of hexachlorocyclotriphosphazene (HCCP) into 30ml of dioxane, and ultrasonically disperse at room temperature to obtain a uniform solution; take 4.011g of N-aminoethylpiperazine (AEP) and 3.143g of tris Ethylamine (TEA) was added to a three-necked flask filled with 20ml of dioxane; the molar ratio of HCCP and AEP was controlled to be 1:6. The solution of hexachlorocyclotriphosphazene was dropped into a three-necked flask filled with triethylamine and N-aminoethylpiperazine using a constant pressure dropping funnel, and reacted ultrasonically at 35° C. for 5 h. After the reaction was completed, after cooling to room temperature, the product was centrifuged, washed three times with dioxane and deionized water, and the obtained solid product was placed in a blast drying oven at a drying temperature of 100°C. Dry for 24 hours to obtain active amino cross-linked phosphazene derivatives.
Embodiment 3
[0056] Add 1.7383g of hexachlorocyclotriphosphazene (HCCP) into 30ml of dioxane, and ultrasonically disperse at room temperature to obtain a uniform solution; take 2.584g of N-aminoethylpiperazine (AEP) and 2.0238g of tris Ethylamine (TEA) was added to a three-necked flask filled with 20ml of dioxane; the molar ratio of HCCP and AEP was controlled to be 1:4. The solution of hexachlorocyclotriphosphazene was dripped into a three-necked flask filled with triethylamine and N-aminoethylpiperazine using a constant pressure dropping funnel, and reacted ultrasonically at 30° C. for 5 h. After the reaction was completed, after cooling to room temperature, the product was centrifuged, washed three times with dioxane and deionized water, and the obtained solid product was placed in a blast drying oven at a drying temperature of 100°C. Dry for 24 hours to obtain active amino cross-linked phosphazene derivatives.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com