New compound bis-(4-aminophenyl) phenyl phosphonate and synthesis method thereof
A technology of phenylphosphonate and aminophenyl, which is applied in the field of compound di-phenylphosphonate and its synthesis, can solve problems such as easy fire and restriction, and achieves that it is not easy to migrate and exude, and has little impact on performance. , the effect of permanent flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
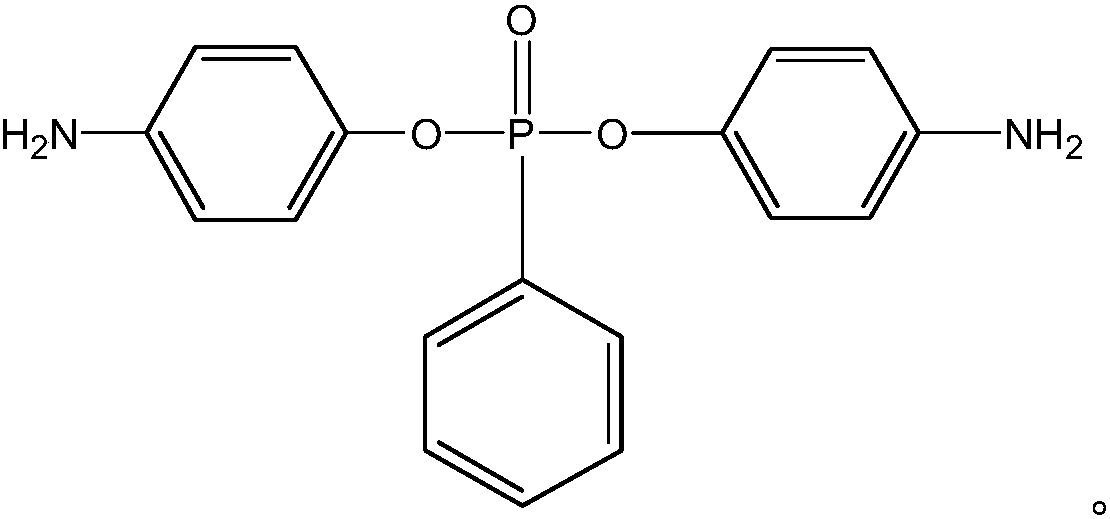
[0030] A kind of compound bis-(4-aminophenyl) phenyl phosphonate, its molecular structural formula is as follows:
[0031]
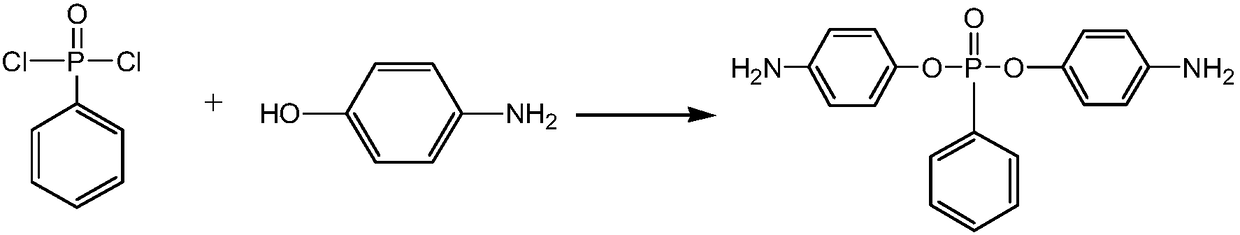
[0032] Compound bis-(4-aminophenyl) phenyl phosphonate synthetic method, the steps are as follows:
[0033] 1) Weigh p-aminophenol (2.18g 0.02mol) and phenylphosphonic dichloride (1.95g0.01mol) at a molar ratio of 2:1, and weigh Cu according to the amount of catalyst added as 1wt% of the p-aminophenol mass 2 Cl 2 (0.02g).
[0034] 2) Under an inert gas atmosphere, add a dropping funnel, a stirrer, anhydrous CaCl 2 Add p-aminophenol, 3ml triethylamine and 20ml tetrahydrofuran to the condensing reflux device of the protective tube, and cool to 0°C in an ice-water bath. Then add Cu 2 Cl 2 , Dissolve phenylphosphonic dichloride in 10ml tetrahydrofuran, add dropwise within 1.5h, and react at 30°C for 24h. The final product was obtained after filtration, precipitation and recrystallization.
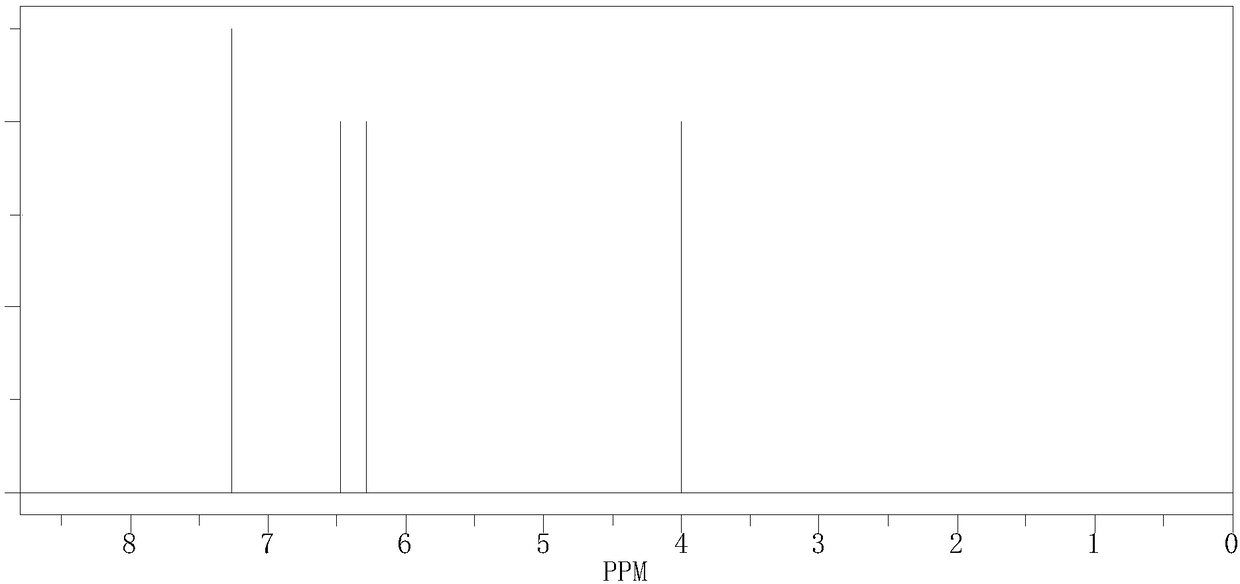
[0035] figure 1 As a new curing agent bis-(4-aminophenyl)...
Embodiment 2
[0039] Compound two-(4-aminophenyl) phenyl phosphonate, its synthetic method is as follows:
[0040] 1) Weigh p-aminophenol (2.18g 0.02mol) and phenylphosphonic dichloride (1.95g0.01mol) at a molar ratio of 2:1, and weigh Cu according to the amount of catalyst added as 1.5wt% of the p-aminophenol mass 2 Cl 2 (0.03g).
[0041] 2) Under an inert gas atmosphere, add a dropping funnel, a stirrer, anhydrous CaCl 2 Add p-aminophenol, 3ml triethylamine and 20ml tetrahydrofuran to the condensing reflux device of the protective tube, and cool to 0°C in an ice-water bath. Then add Cu 2 Cl 2 , Dissolve phenylphosphonic dichloride in 10ml tetrahydrofuran, add dropwise within 1h, and react at 30°C for 24h. The final product was obtained after filtration, precipitation and recrystallization.
Embodiment 3
[0043] Compound, its synthetic method is as follows:
[0044] 1) Weigh p-aminophenol (2.18g 0.02mol) and phenylphosphonic dichloride (1.95g0.01mol) at a molar ratio of 2:1, and weigh Cu according to the amount of catalyst added as 2wt% of the p-aminophenol mass 2 Cl 2 (0.04g).
[0045] 2) Under an inert gas atmosphere, add a dropping funnel, a stirrer, anhydrous CaCl 2 Add p-aminophenol, 3ml triethylamine and 20ml tetrahydrofuran to the condensing reflux device of the protective tube, and cool to 0°C in an ice-water bath. Then add Cu 2 Cl 2, Dissolve phenylphosphonic dichloride in 10ml tetrahydrofuran, add dropwise within 2h, and react at 30°C for 24h. The final product was obtained after filtration, precipitation and recrystallization.
PUM
| Property | Measurement | Unit |
|---|---|---|
| limiting oxygen index | aaaaa | aaaaa |
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com