Novel use and preparation method of human beta-defensin 3
A technology for defensins and new uses, which is applied in the field of new uses and preparations of active small molecular proteins, and can solve the problems of difficulty in ensuring the biological activity of polypeptides, high cost, and low yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0027] Experimental example 1: Construction experiment of expressing engineered bacteria Rosseta-gami(2)-pET32a(+) / HBD3
[0028] 1. Construction of pET32a(+) / HBD3 recombinant plasmid
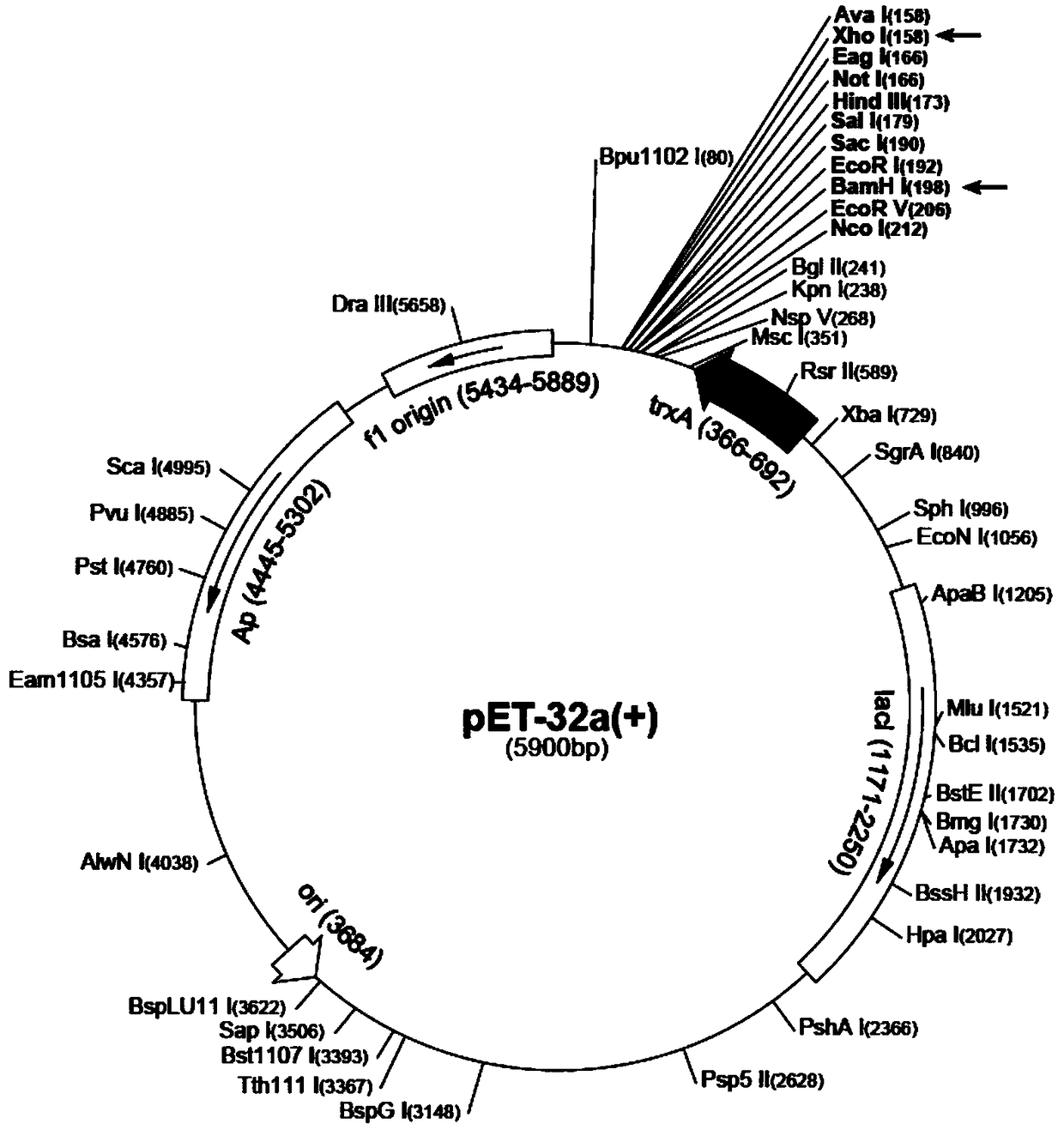
[0029] (1) Extraction of plasmid pET32a(+): Culture Escherichia coli JM109 (containing pET32a(+) plasmid) by shaking with LB medium, collect bacterial cells, and operate with a plasmid extraction kit to obtain pET32a(+) plasmid (pET32a (+) The schematic diagram of the plasmid structure is shown in figure 1 ).
[0030] (2) Restriction endonuclease reaction: The synthesized HBD3 mature peptide gene and pET32a(+) plasmid were digested with BamHI and XholⅠ under appropriate conditions, and the reaction products were gel-recovered after agarose electrophoresis to obtain enzyme digestion product.
[0031] (3) Ligation reaction: The above-mentioned digested products were recovered and purified by gel, and then ligation reaction was carried out. Under the action of T4 DNA ligase reaction, the molecu...
experiment example 2
[0040] Experimental example 2: Induced expression, identification and optimization of induction conditions of HBD3 fusion protein
[0041] 1. Induced expression and identification of HBD3 fusion protein
[0042] (1) Induced expression of HBD3 fusion protein: the glycerol-preserved expression strains Rossetta-gami(2)-pET32a(+) / HBD3 and Rossetta-gami(2)-pET32a(+) were inoculated in 5 mL LB / AMP liquid medium respectively, Shake culture at 37°C overnight, take 50 μL of bacterial solution and re-inoculate 5 mL of LB / AMP liquid medium at an inoculum size of 1:100, shake at 37°C and 200 rpm for 4 hours, until the bacteria reach the logarithmic growth phase (A600≈0.6) , as a positive control, no IPTG was added, and the rest were added with IPTG to a final concentration of 0.5mM, then continued to culture at 37°C for 4h, centrifuged to remove the supernatant, washed the bacterial cells twice with 0.01mol / LPBS buffer 1mL, and then added 0.5mL of PBS Resuspended cells.
[0043] (2) SDS...
experiment example 3
[0055] Experimental example 3: Purification of recombinant HBD3 polypeptide
[0056] 1. Affinity chromatography purification of HBD3 fusion protein
[0057] (1) Inoculate the glycerin-preserved expression strain Rossetta-gami(2)-pET32a(+) / HBD3 in 5 mL LB / AMP liquid medium, culture with shaking at 37°C overnight, take 50 μL of bacterial liquid and re-inoculate with an inoculum volume of 1:100 In 1L LB / AMP liquid medium, shake culture at 37°C and 200rpm for 4h, until the bacteria reach the logarithmic growth phase (A600≈0.6), add IPTG to a final concentration of 0.5mM, then continue to culture at 34°C for 6h, centrifuge to remove After the supernatant, wash the bacterial cells twice with 1 mL of 0.01 mol / LPBS buffer, add the bacterial cells to the ratio of 10 mL of culture to 1 mL of Binding Buffer, and resuspend the bacterial cells.
[0058] (2) Put the supernatant on ice and sonicate (probe No. 6, 100 power, sonicate for 3 s, interval 3 s, act for 30 min), centrifuge at 4°C a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com