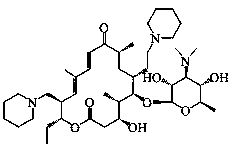
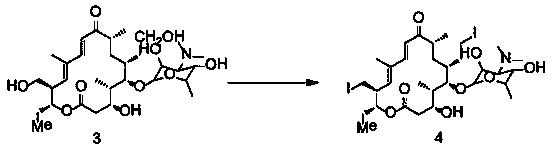Synthetic method of Tildipirosin intermediate
A technology of tediloxine and synthesis method, which is applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of low product purity, high iodine activity, and complicated purification process, and achieve product purity. High, simple separation process, high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Synthesis of 20-piperidinyl-23-iodo-5-O-mycaminosyl-tylonolide
[0044] In a 500L reactor, put 71.5kg of 20-piperidinyl-5-O-mycaminosyl-tylonolide (1) and 160kg of acetonitrile into stirring, add 44.1kg of hydroiodic acid (57%), and reflux reaction After 4.5 hours (the reaction kettle is connected to the tail gas absorption tank), concentrate under reduced pressure until there is almost no solvent, add water, adjust the pH to 11 with sodium hydroxide solution, stir for 20 minutes, filter with suction, and dry to obtain the product 20-piperidinyl- 23-iodo-5-O-mycaminosyl-tylonolide (2) 63.0kg, yield 95.4%, purity 98.1%.
Embodiment 2
[0045] Example 2 Synthesis of 20-piperidinyl-23-iodo-5-O-mycaminosyl-tylonolide
[0046] In a 500L reactor, put 71.5kg of 20-piperidinyl-5-O-mycaminosyl-tylonolide (1) and 160kg of acetonitrile into stirring, add 44.1kg of hydroiodic acid (57%), and reflux reaction After 4.5 hours (the tail gas absorption tank is connected to the reaction kettle), concentrate under reduced pressure to almost no solvent, add water, adjust the pH to 12 with sodium hydroxide solution, stir for 20 minutes, filter with suction, and dry to obtain the product 20-piperidinyl- 23-iodo-5-O-mycaminosyl-tylonolide (2) 63.2kg, yield 95.8%, purity 98.3%.
Embodiment 3
[0047] Example 3 Synthesis of 20-piperidinyl-23-iodo-5-O-mycaminosyl-tylonolide
[0048] In a 500L reactor, put 71.5kg of 20-piperidinyl-5-O-mycaminosyl-tylonolide (1) and 160kg of acetonitrile and stir, add 39.5kg of hydroiodic acid (57%), and reflux the reaction After 4.5 hours (the tail gas absorption tank is connected to the reaction kettle), concentrate under reduced pressure to almost no solvent, add water, adjust the pH to 12 with sodium hydroxide solution, stir for 20 minutes, filter with suction, and dry to obtain the product 20-piperidinyl- 23-iodo-5-O-mycaminosyl-tylonolide (2) 62.9kg, yield 95.3%, purity 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com