A kind of concentration method of virus liquid
A technology of virus liquid and virus, which is applied in the concentration of virus liquid and the field of virus culture, can solve the problems of inability to obtain virus, discomfort of virus particles, and harmfulness of infected cells. foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Cell subculture: Take out 1 tube of frozen ST cells from liquid nitrogen, thaw quickly in a 37°C water bath, use a sterile pipette to draw 1.0ml of cell suspension into a 15ml sterile centrifuge tube, and add drop by drop MEM medium containing 10wt% FBS to 5-15mL, pipet several times, mix evenly, centrifuge immediately (3000rpm, 5min), and discard the supernatant. Then add 10-20mL of 10wt% FBS MEM medium, pipette several times, mix well, transfer to T25 culture bottle with 10wt% FBS MEM medium, place at 37°C, 5% CO 2 Cultivate in an incubator for 48h to 72h, and when the cells cover a monolayer, set aside.
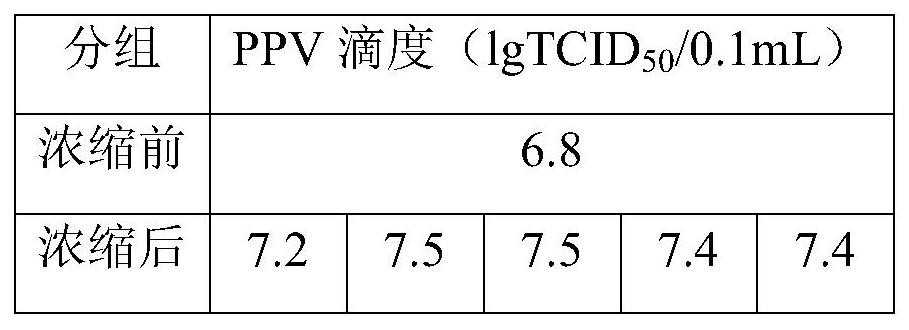
[0023] 2. Subculture of the virus: Take out 1 tube of frozen PPV virus from the -70°C refrigerator (working virus bank), redissolve in a 37°C water bath, dilute 10 times with MEM medium without FBS, and then inoculate 1mL in In the cell bottle that has been covered with a monolayer of cells, store at 37°C, 5% CO 2 The incubator was adsorbed for 1 hour, and the ...
Embodiment 2
[0029] 1. Cell subculture: Take out 1 tube of frozen LLC-MK2 cells from liquid nitrogen, thaw quickly in a 37°C water bath, use a sterile pipette to draw 1.0ml of cell suspension into a 15ml sterile centrifuge tube, drop by drop Add dropwise DMEM medium containing 10wt% FBS to 5-15mL, pipette several times, mix well, centrifuge immediately (3000rpm, 5min), and discard the supernatant. Then add 10-20 mL of DMEM medium with 10wt% FBS, pipette several times, mix well, transfer to T25 culture bottle with DMEM medium with 10wt% FBS, place at 37°C, 5% CO 2 Cultivate in an incubator for 24h to 48h, and when the cells cover a single layer, set aside.
[0030] 2. Subculture of the virus: take out a tube of frozen ReoV-3 virus from the -70°C refrigerator (working virus bank), redissolve it in a 37°C water bath, dilute it 10 times with MEM medium without FBS, and then pipette 1mL Inoculate in a cell bottle that has been filled with a monolayer of cells, store at 37°C, 5% CO 2 The incub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com