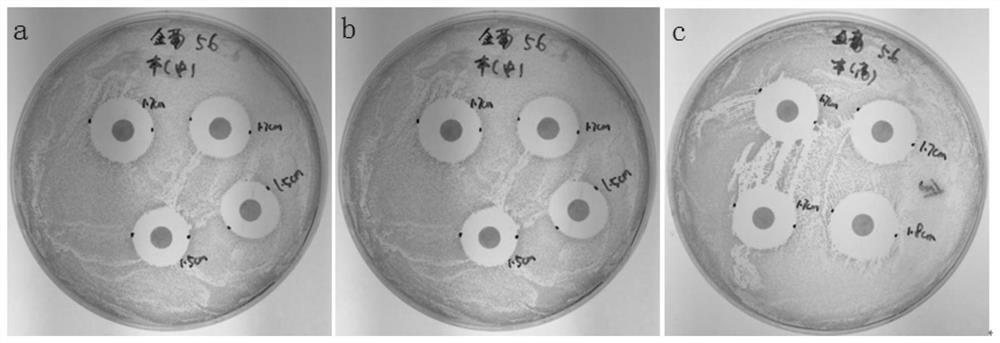
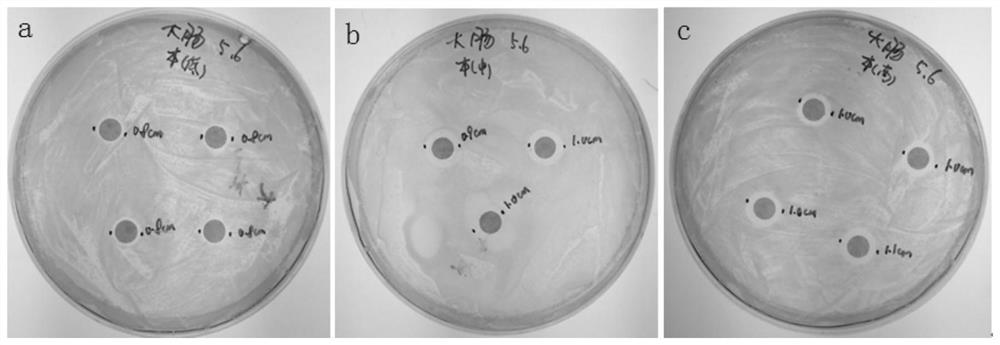
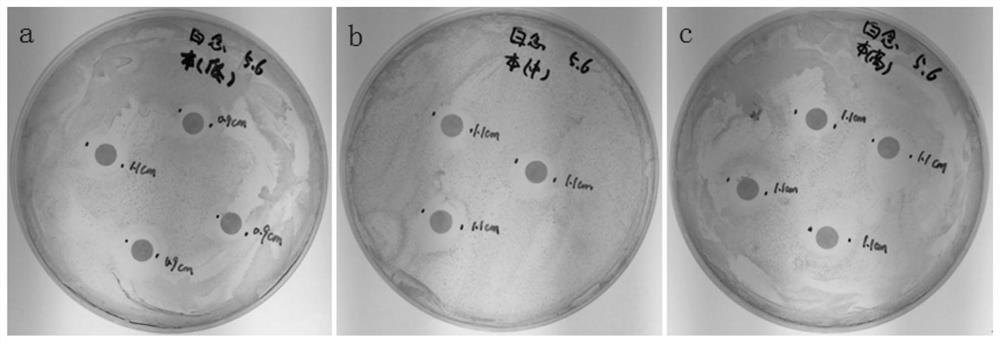
Virus transport medium and its preparation method and application
A medium and virus technology, applied in the field of culture medium, can solve the problems of uneven performance and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A virus transport culture medium is prepared by the following method:
[0036] 1) Prepare 4× Hank’s balanced salt solution according to the following formula:
[0037]
[0038] 2) Prepare the virus delivery medium according to the table below:
[0039] Table 1. The virus delivery medium preparation table of embodiment 1
[0040] component name stock solution concentration Dosage Final concentration Hank's Balanced Salt Solution 4× 125mL 0.5× gelatin 5% 20mL 1g / L BSA 10% 25mL 2.5g / L sucrose 5% 20mL 1g / L L-cysteine 0.015mmol / mL 6.6mL 0.1mM / L L-glutamic acid 0.015mmol / mL 6.6mL 0.1mM / L Hepes 1M / L 5mL 5mM / L amphotericin B 2.5mg / mL 4mL 10mg / L Polymyxin B 2mg / mL 5mL 10mg / L Gentamicin 10mg / mL 1mL 10mg / L water / Fixed volume to 1L /
[0041] Among them, 4 × Hank's balanced salt solution, sucrose, BSA solution, L-cysteine, L-glutamic acid, Hepe...
Embodiment 2
[0043] A virus delivery medium, the difference from Example 1 is that the components and proportioning ratio are shown in the following table:
[0044] Table 2. The virus delivery medium preparation table of embodiment 2
[0045] component name stock solution concentration Dosage (to prepare 1L) Final concentration Hank's Balanced Salt Solution 4× 250mL 1× gelatin 5% 50mL 2.5g / L BSA 10% 50mL 5g / L sucrose 5% 50mL 2.5g / L L-cysteine 0.015mmol / mL 10mL 0.150mM / L L-glutamic acid 0.015mmol / mL 10mL 0.150mM / L Hepes 1M 10mL 10mM / L amphotericin B 2.5mg / mL 8mL 20mg / L Polymyxin B 2mg / mL 10mL 20mg / L Gentamicin 10mg / mL 2mL 20mg / L water / Fixed volume to 1L /
Embodiment 3
[0047] A virus delivery medium, the difference from Example 1 is that the components and proportioning ratio are shown in the following table:
[0048] Table 3. Example 3 virus delivery medium preparation table
[0049] component name stock solution concentration Dosage (to prepare 1L) Final concentration Hank's Balanced Salt Solution 4× 500mL 2× gelatin 5% 100mL 5g / L BSA 10% 100mL 10g / L sucrose 5% 100mL 5g / L L-cysteine 0.015mmol / mL 20mL 0.3mM / L L-glutamic acid 0.015mmol / mL 20mL 0.3mM / L Hepes 1M 20mL 20mM / L amphotericin B 2.5mg / mL 16mL 40mg / L Polymyxin B 2mg / mL 20mL 40mg / L Gentamicin 10mg / mL 4mL 40mg / L water / Fixed volume to 1L /
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com