Novel method for synthesis and purification of aclidinium bromide
A kind of technology of aclidinium bromide and new method, applied in the field of organic compound synthesis and purification, can solve problems such as difficult control, low yield, harsh reaction conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
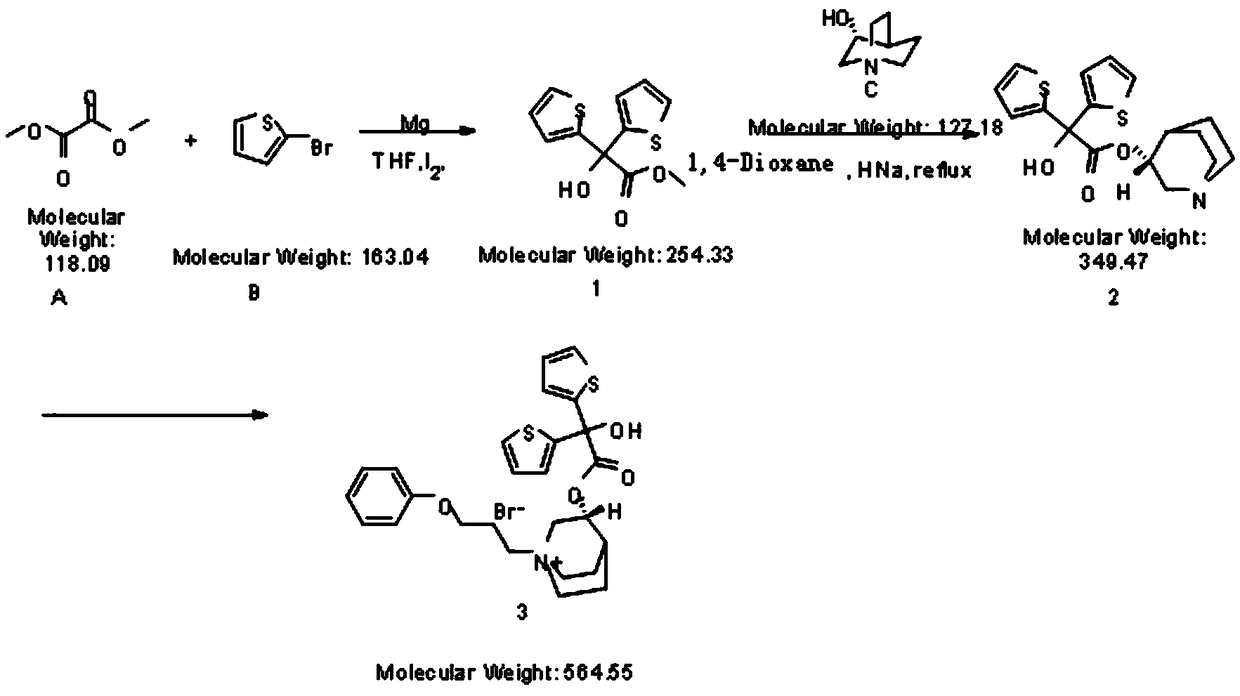
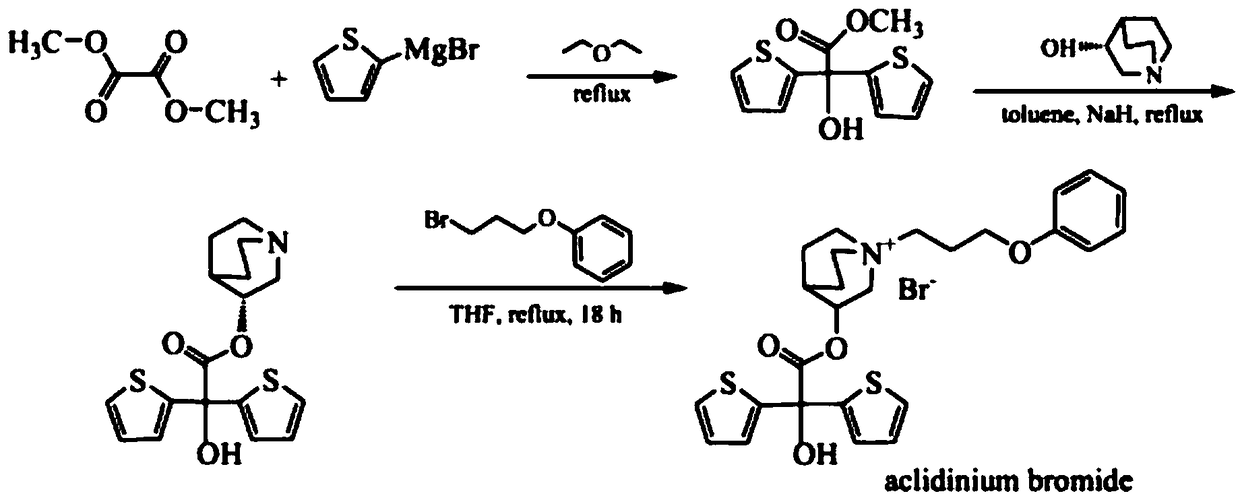
[0036] The invention provides a new method for the synthesis and purification of aclidinium bromide, the synthesis and purification method comprising the following steps:
[0037] Magnesium powder (30g, 1250mmol, 2.5eq), iodine (0.5g), THF (600mL) were added into a 1L three-necked flask, and 10mL of 2-bromothiophene (179g, 1100mmol, 2.2q) was added dropwise under heating and reflux conditions. THF (100 mL) mixed solution, and then bromoethane (1 mL) was added dropwise into a 1 L three-necked flask to control the reaction rhythm. Control the dropping rate, keep the reaction violently reflux, and reflux for about 1 hour after the addition to prepare the Grignard reagent;
[0038] Add dimethyl oxalate (59g, 500mmol, 1eq) and THF (360mL) into a 2L three-necked flask, cool in an ice-water bath to -5°C, add the above-mentioned Grignard reagent dropwise into a 2L three-necked flask, and mix in the temperature-controlled three-necked flask The solution was at -5°C, and the ice-water ...
Embodiment 2
[0040] The invention provides a new method for synthesizing and purifying aclidinium bromide, the preparation method comprising the following steps:
[0041] Magnesium powder (30g, 1250mmol, 2.5eq), iodine (0.5g), THF (600mL) were added into a 1L three-necked flask, and 10mL of 2-bromothiophene (179g, 1100mmol, 2.2q) was added dropwise under heating and reflux conditions. THF (100 mL) mixed solution, and then bromoethane (1 mL) was added dropwise into a 1 L three-necked flask to control the reaction rhythm. Control the dropping rate, keep the reaction violently reflux, and reflux for about 1 hour after the addition to prepare the Grignard reagent;
[0042] Add dimethyl oxalate (59g, 500mmol, 1eq) and THF (360mL) to a 2L three-necked flask, cool in an ice-water bath to -4°C, then add the above-mentioned Grignard reagent dropwise into a 2L three-necked flask, and mix in the temperature-controlled three-necked flask The solution was at -4°C, and the ice-water bath was removed afte...
Embodiment 3
[0044] The invention provides a new method for synthesizing and purifying aclidinium bromide, the preparation method comprising the following steps:
[0045] Magnesium powder (30g, 1250mmol, 2.5eq), iodine (0.5g), THF (600mL) were added into a 1L three-necked flask, and 10mL of 2-bromothiophene (179g, 1100mmol, 2.2q) was added dropwise under heating and reflux conditions. THF (100 mL) mixed solution, and then bromoethane (1 mL) was added dropwise into a 1 L three-necked flask to control the reaction rhythm. Control the dropping rate, keep the reaction violently reflux, and reflux for about 1 hour after the addition to prepare the Grignard reagent;
[0046] Add dimethyl oxalate (59g, 500mmol, 1eq) and THF (360mL) into a 2L three-necked flask, cool in an ice-water bath to -3°C, then add the above-mentioned Grignard reagent dropwise into a 2L three-necked flask, and mix in the temperature-controlled three-necked flask The solution was at -3°C, and the ice-water bath was removed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com