Preparation method of barium carbonate and barium carbonate prepared by using same
A technology of barium carbonate and barium sulfide, applied in the direction of barium carbonate, calcium carbonate/strontium/barium, etc., can solve the problems of reducing costs and solid waste, and achieve the effects of reducing treatment costs, large environmental benefits, and multiple economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
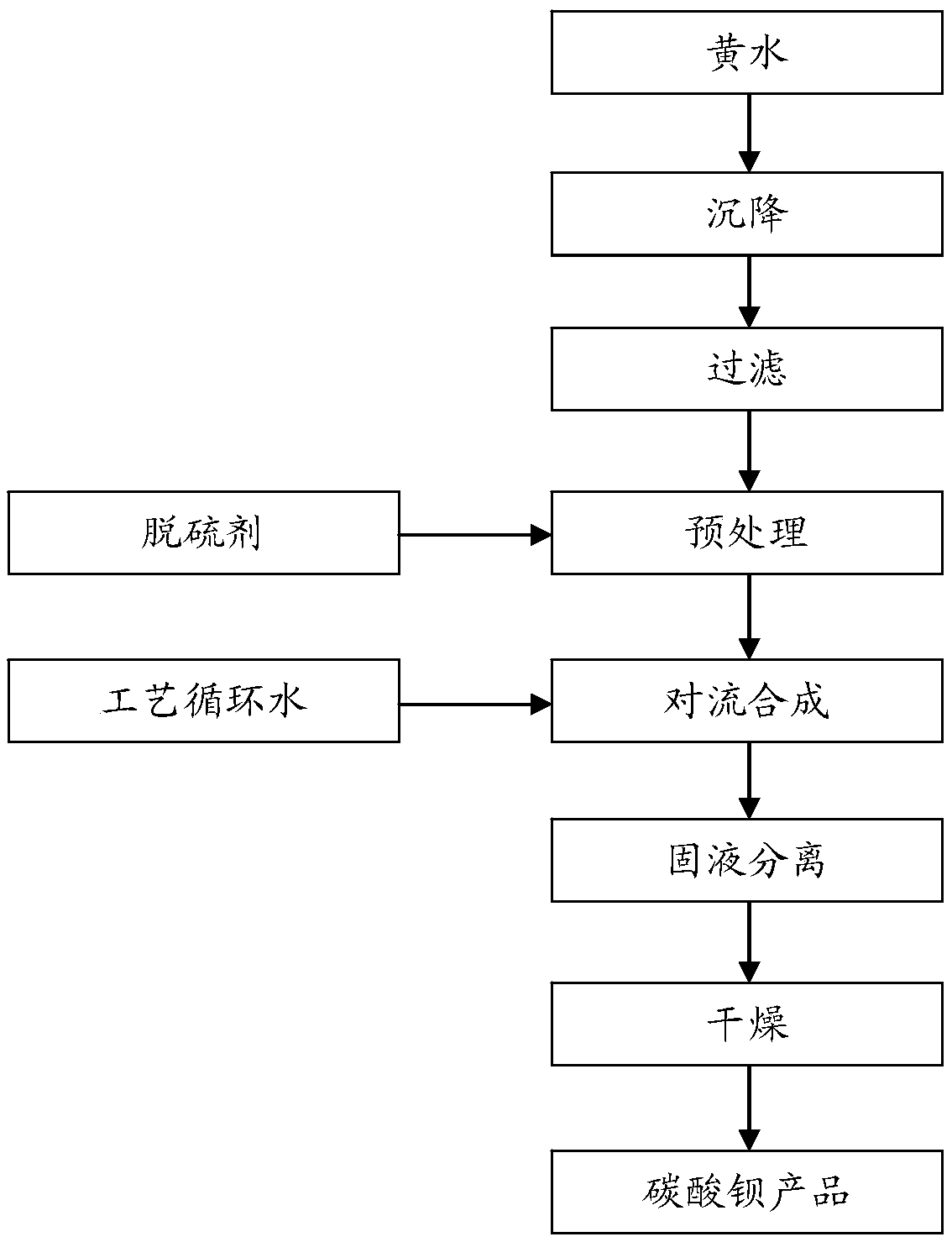
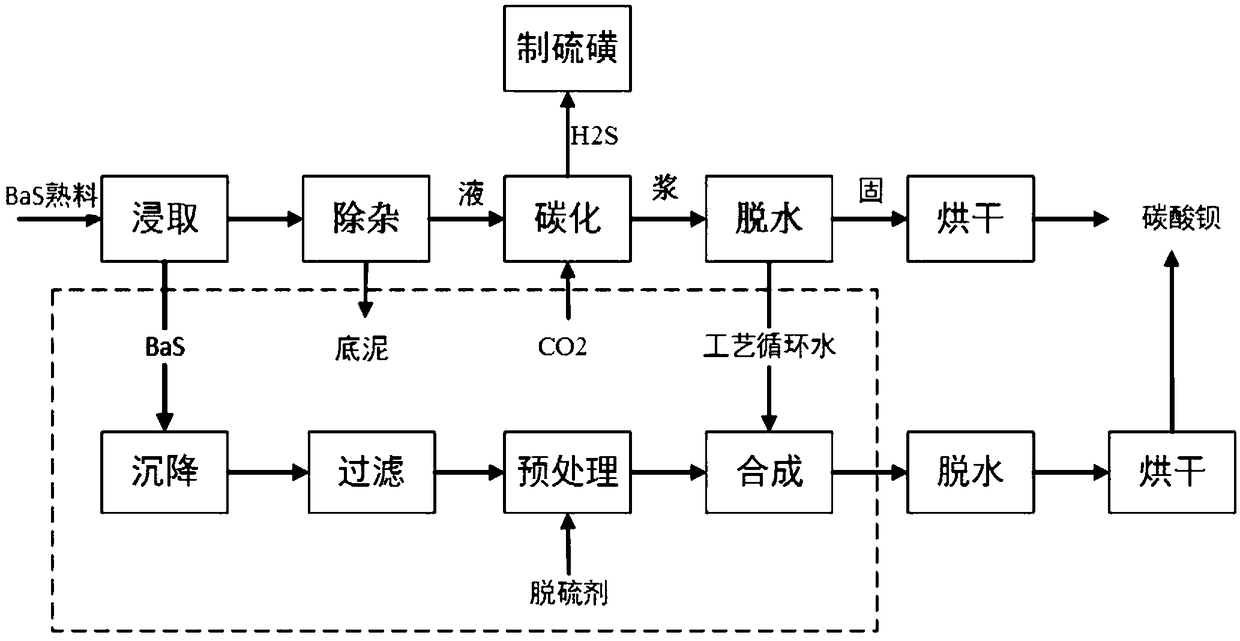
[0032] Barium carbonate preparation method of the present invention comprises:
[0033] The first step is to purify low-concentration yellow water: settle the yellow water and press filter to obtain pure yellow water.
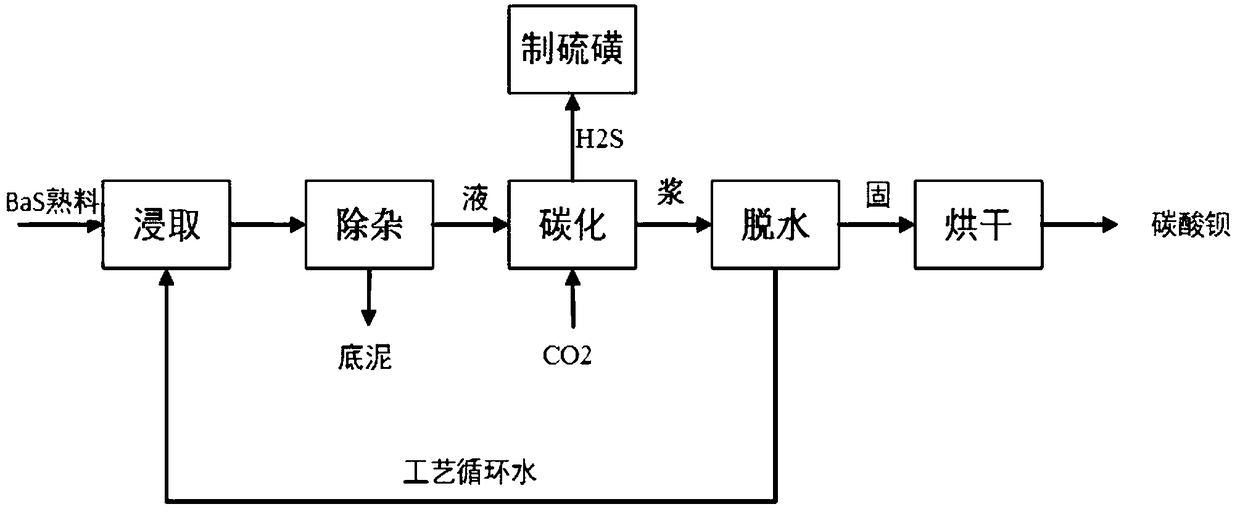
[0034] In the present invention, yellow water refers to the leaching solution that barite reduction roasting produces BaS clinker, and its main component is Ba(OH) 2 、Ba(HS) 2 , the concentration of yellow water mentioned in the art is usually represented by barium sulfide concentration, which is just a common expression in the art.
[0035] Specifically, the leached low-concentration yellow water is poured into the low-concentration yellow water clarification tank, and the purpose of clarification is achieved through natural sedimentation. Because it also contains fine impure suspended matter, it is filtered through a plate and frame filter press to obtain pure yellow water. . After sedimentation and pressure filtration, impurities such as insoluble matter ...
Embodiment 1
[0056] (1) Put the yellow water into the low-concentration yellow water clarification tank, achieve the purpose of clarification by natural sedimentation, and then press filter through a plate and frame filter press to remove fine impure suspended matter, and obtain pure low-concentration yellow water, which is a concentration of 50g / L BaS solution.
[0057] (2) Add 0.3mol / L sodium hydroxide solution to pure yellow water with a constant flow pump to control Na + When the concentration is 0.015mol / L, stop the pretreatment.
[0058] (3) The pretreated BaS solution and the process circulating water are subjected to the addition reaction, and the flow rates of the BaS solution and the process circulating water are respectively 3m 3 / h and 15m 3 / h, the reaction time is 15 minutes, the pH value of the reaction end point is controlled to be 11, and then it is naturally settled for 2 hours. After completion, the slurry is filtered, the solid is collected, and the solution is retain...
Embodiment 2
[0061] (1) Put the yellow water into the low-concentration yellow water clarification tank, achieve the purpose of clarification by natural sedimentation, and then press filter through a plate and frame filter press to remove fine impure suspended matter, and obtain pure low-concentration yellow water, which is a concentration of 30g / L BaS solution.
[0062] (2) Add 0.1mol / L sodium hydroxide solution to pure yellow water with a constant flow pump to control Na + When the concentration is 0.01mol / L, stop the pretreatment.
[0063] (3) The pretreated BaS solution and the process circulating water are subjected to the addition reaction, and the flow rates of the BaS solution and the process circulating water are respectively 3m 3 / h and 10m 3 / h, the reaction time is 30 minutes, the pH value of the reaction end point is controlled to be 9.5, and then it is naturally settled for 5 hours. After completion, the slurry is filtered, the solid is collected, and the solution is retain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com