Targeted disruption of the t cell receptor
A cell receptor and cell technology, applied in the field of human cell genome modification, which can solve the problem of inability to generate graft-versus-host disease cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
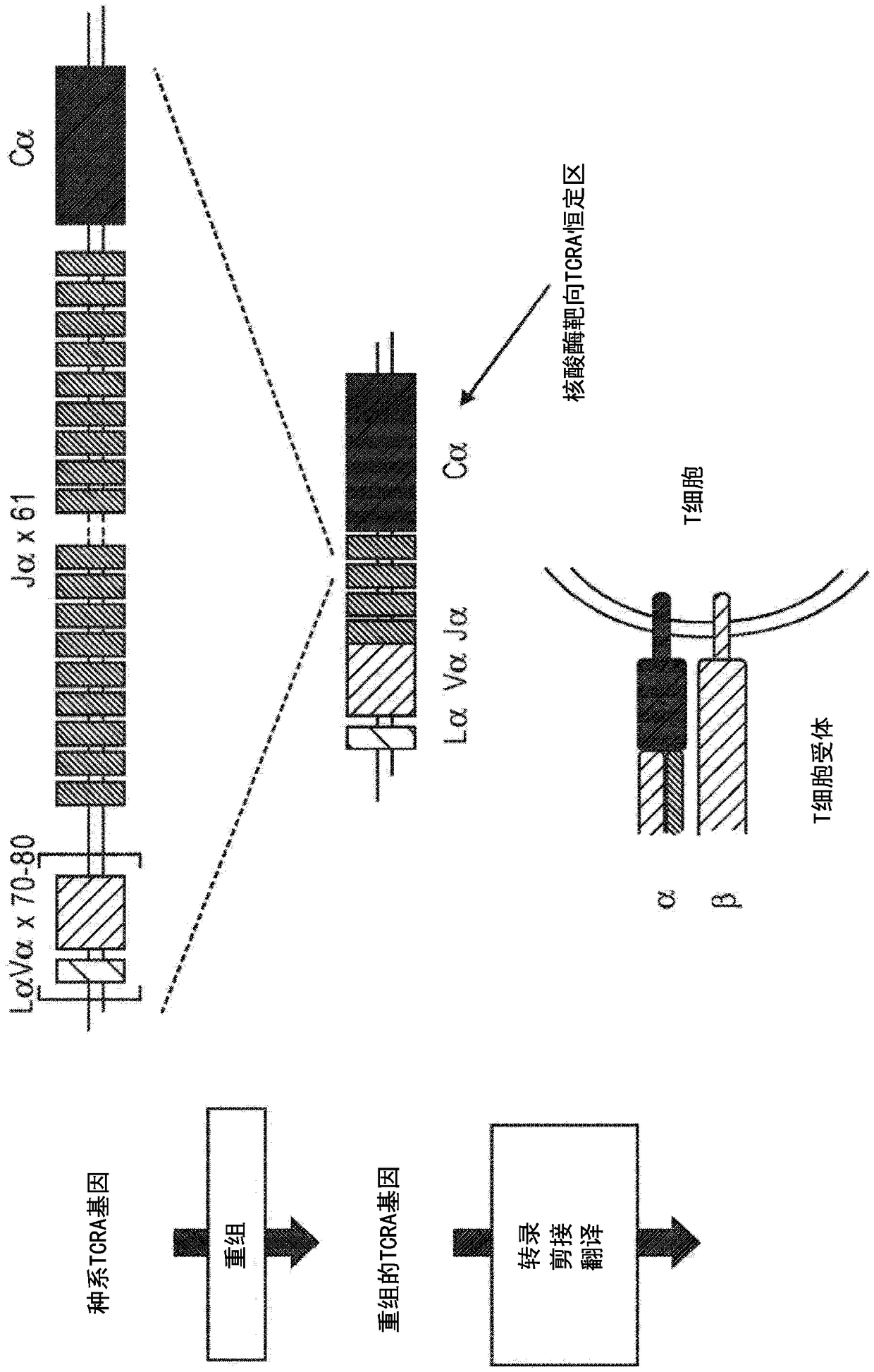
[0192] Example 1: Design of TCR-specific nucleases
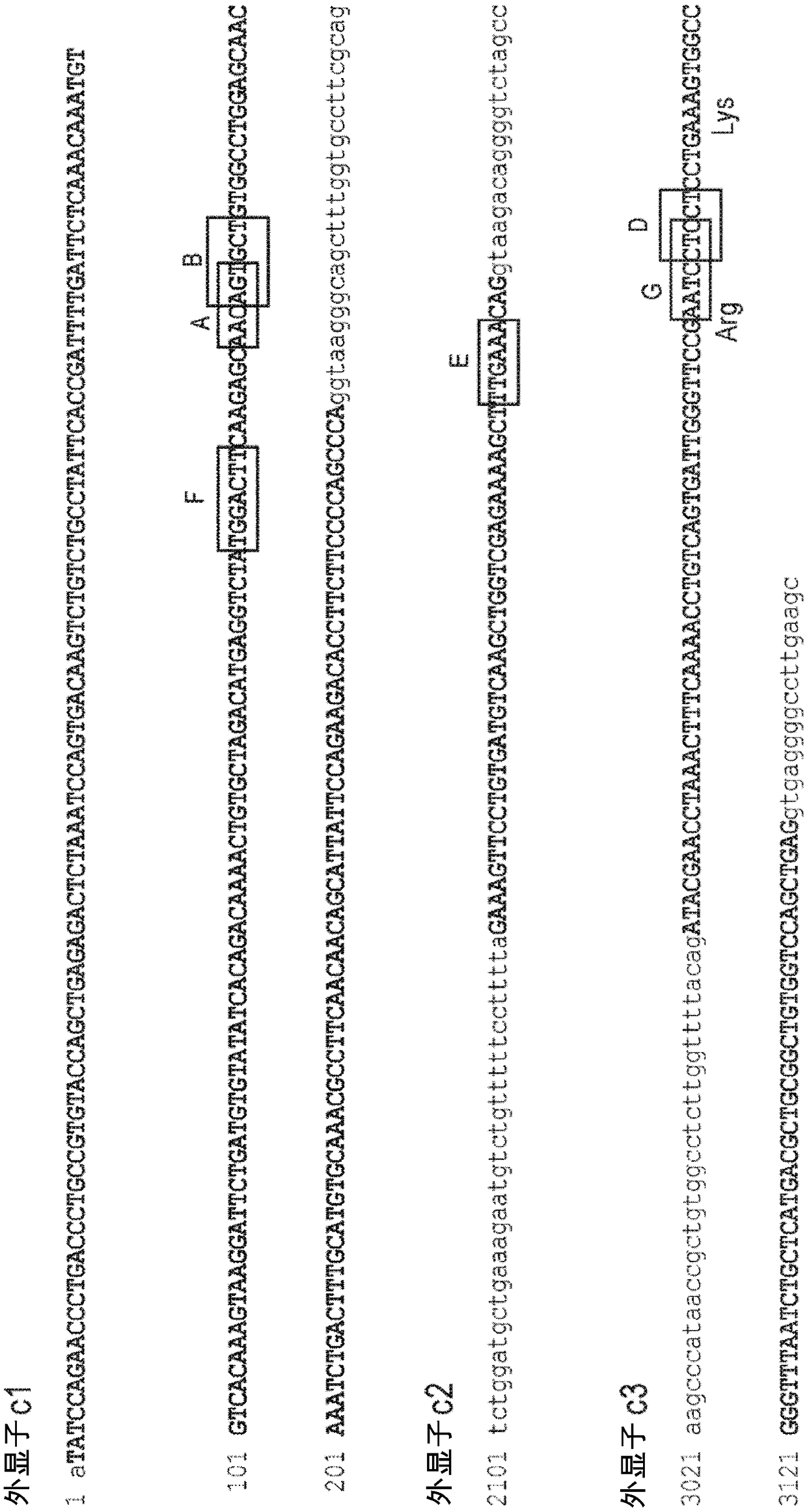
[0193] TCR-specific ZFNs were constructed to achieve site-specific introduction of double-strand breaks located at the TCRα (TCRA) gene. Principally by Urnov et al. (2005) Nature 435(7042):646-651, Lombardo et al. (2007) Nat Biotechnol. Nov; 25(11):1298-306, and U.S. Patent Publication Nos. 2008-0131962, 2015-016495 , 2014-0120622, 2014-0301990 and US Pat. No. 8,956,828 to design ZFNs. ZFN pairs target different sites in the constant region of the TCRA gene (see Figure 1). The recognition helices and target sequences for exemplary ZFN pairs are shown in Table 1 below. The target sites of TCRA zinc finger design are shown in the first column. Nucleotides in the target site targeted by the ZFP recognition helix are indicated in upper case; nucleotides not targeted are indicated in lower case. The linker used to connect the FokI nuclease domain and the ZFP DNA binding domain is also shown (see, US Patent Publication No. 201...
Embodiment 2
[0204] Embodiment 2: in vitro nuclease activity
[0205] The ZFNs described in Table 1 were used to test nuclease activity in K562 cells. To test for cleavage activity, plasmids encoding the above human TCRA-specific ZFN pairs were transfected into K562 cells together with plasmids or mRNA. K562 cells were obtained from the American Type Culture Collection and grown as recommended in RPMI medium (Invitrogen) supplemented with 10% quantitative fetal bovine serum (FBS, Cy clone). For transfection, the ORFs of the active nucleases listed in Table 1 were cloned into expression vectors optimized for mRNA production containing 5' and 3' UTRs and a synthetic poly-A signal. mRNA was generated using the mMessage mMachine T7 Ultra kit (Ambion) following the manufacturer's instructions. In vitro synthesis of nuclease mRNA using a pVAX-based vector containing a T7 promoter, an appropriate nuclease, and a poly-A motif, or a pGEM-based vector, or a PCR amplicon for in vitro The poly-A ta...
Embodiment 3
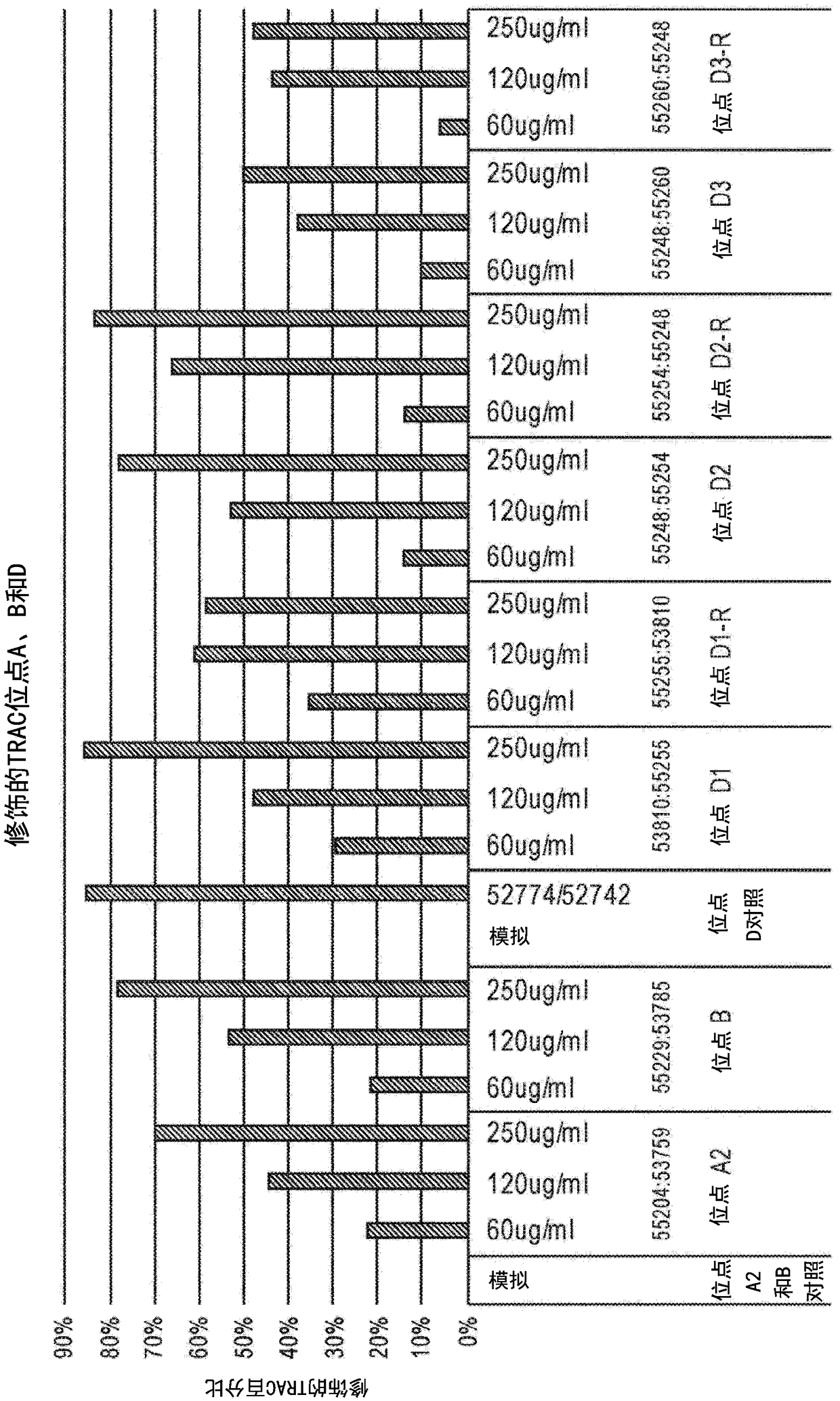
[0217] Example 3: TCRA-specific ZFN activity in T cells
[0218]The nuclease activity of TCRA-specific ZFN pairs was also tested in human T cells. ZFN-encoding mRNAs were transfected into purified T cells. Briefly, T cells were obtained from leukopheresis products and purified using Miltenyi's CliniMACS system (CD4 and CD8 dual selection). These cells were then activated using Dynabeads (ThermoFisher) according to the manufacturer's protocol. Three days after activation, cells were transfected with three doses of mRNA (60, 120 and 250 μg / mL) using a Maxcyte electrotransformer (Maxcyte Company), OC-100, 30e6 cells / mL, and a volume of 0.1 mL. On day 10 after transfection, cells were analyzed for ontarget TCRA modification using deep sequencing (Miseq, Illumina). Cell viability and cell growth (total cell doublings) were measured throughout 13-14 days in culture. In addition, at day 10 of culture for CD3 staining, TCR was measured on the cell surface of treated cells using st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com