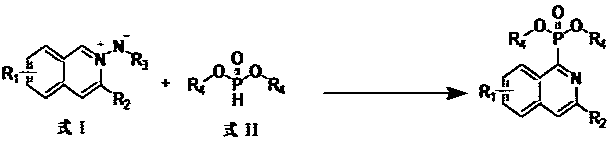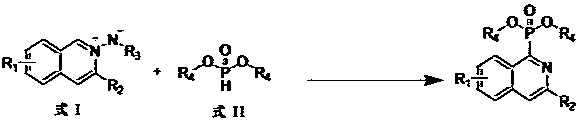Preparation method of isoquinoline phosphite ester compound
A technology of isoquinoline phosphite and compounds, which is applied in the direction of phosphorus organic compounds, can solve the problems that are not conducive to the promotion and popularization of industrialization, the production rate is not very high, and the production cost is increased, so as to achieve high utilization rate of atom economy and low production cost. Low, productivity-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 32.0 mg (0.1 mmol) Tert-butoxycarbonyl (3-phenyl isoquinolinium-2-yl) amide (R1 = hydrogen, R2 = phenyl, R3 = tert-butyryl, R4 = ethyl), 27.6 mg (0.2 mmol) Diethyl phosphate, 27.6 mg (0.2 mmol) K 2 CO 3 , 1.9 mg (0.01 mmol) CuI was added to the reaction test tube, and then 2 mL of DCE (1,2 dichloroethane) was added, at 120 o The crude product was obtained by heating and reacting at C for 24 hours, cooled after the reaction, filtered, the filtrate was rotary evaporated, and the solvent was removed. The residue was subjected to silica gel column chromatography, washed with petroleum ether, and detected by TLC. The solvent was distilled off, and dried in vacuo to obtain 28.0 mg of diethyl (3-phenylisoquinolin-1-yl) phosphonate as a light yellow liquid, with a yield of 82%. 1 H NMR (500 MHz, CDCl 3 ) δ: 8.92 (d, J = 8.5 Hz,1H), 8.15(m, 3H), 7.86 (d, J = 8.0 Hz,1H), 7.64 (dt, J = 15.0, 7.3 Hz, 2H), 7.49 (t, J = 7.5 Hz,2H), 7.40 (t, J = 7.5 Hz,1H), 4.37-4.43 (m, 4...
Embodiment 2
[0018] 33.4 mg (0.1 mmol) (tert-butoxycarbonyl)(3-(p-tolyl)isoquinolin-2-ium-2-yl)amide (R1=hydrogen, R2=p-methylphenyl, R3=tert-butyryl, R4=ethyl), 27.6 mg (0.2 mmol) Diethyl phosphite, 27.6 mg (0.2 mmol) K 2 CO 3 , 1.9 mg (0.01 mmol) CuI was added to the reaction test tube, then 2 mL of DCE was added, at 120 o The crude product was obtained by heating and reacting at C for 24 hours, cooled after the reaction, filtered, the filtrate was rotary evaporated, and the solvent was removed. The residue was subjected to silica gel column chromatography, washed with petroleum ether, and detected by TLC. The solvent was distilled off, and dried in vacuo to obtain 30.2 mg of diethyl (3-(p-tolyl)isoquinolin-1-yl)phosphonate as a pale yellow liquid, with a yield of 85%. 1 H NMR (500 MHz, CDCl 3 ) δ 8.91 (d, J = 8.4 Hz, 1H), 8.13(s, 1H), 8.06 (d, J = 8.0 Hz, 2H), 7.85 (d, J = 8.0 Hz, 1H), 7.64 (dt, J =15.1, 7.2 Hz, 2H), 7.30 (d, J = 7.5 Hz, 2H), 4.59 – 4.22 (m, 4H), 2.41 (s,3H...
Embodiment 3
[0020] 30.0 mg (0.1 mmol) (tert-butoxycarbonyl)(3-butylisoquinolin-2-ium-2-yl)amide (R1=hydrogen, R2=butyl, R3=tert-butyryl, R4=ethyl), 27.6 mg (0.2 mmol) diethyl phosphite, 27.6 mg (0.2 mmol) K 2 CO 3 , 1.9 mg (0.01 mmol) CuI was added to the reaction test tube, and then 2 mL of DCE was added, at 120 oThe crude product was obtained by heating and reacting at C for 24 hours, cooled after the reaction, filtered, the filtrate was rotary evaporated, and the solvent was removed. The residue was subjected to silica gel column chromatography, washed with petroleum ether, and detected by TLC. The solvent was distilled off, and dried in vacuo to obtain 28.0 mg of diethyl (3-butylisoquinolin-1-yl) phosphonate as a pale yellow liquid, with a yield of 80%. 1 H NMR (500 MHz, CDCl 3 ) δ 8.87 (d, J = 8.5 Hz, 1H), 7.77 (d, J = 8.0 Hz,1H), 7.65 (t, J = 7.5 Hz, 1H), 7.62 – 7.51 (m, 2H), 4.56 – 4.15 (m, 4H), 2.99(t, J = 7.6 Hz, 2H), 1.95 – 1.70 (m, 2H), 1.41 (m, 8H), 0.95 (t, J = 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com