Synthesis and application of multifunctional fluorescent molecular probe for simultaneously distinguishing and detecting Cys, Hcy and GSH
A fluorescent molecular probe, a multi-functional technology, applied in the field of analytical chemistry, which can solve problems such as unsatisfactory discrimination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
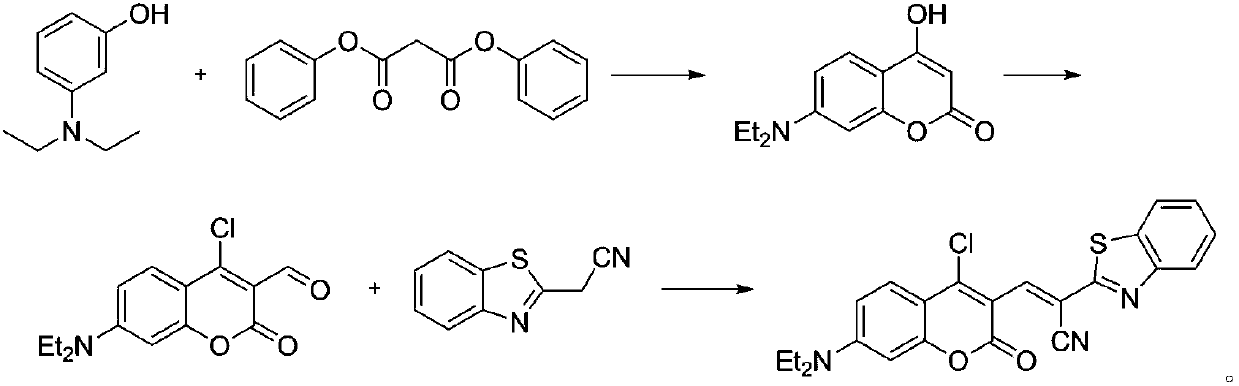
[0024] Example 1. Synthesis Synthesis of 7-(diethylamino)-4-hydroxychromen-2-one
[0025] Add 12.8 g (49.93 mmol) of diphenyl malonate and 8.25 g (49.93 mmol) of 3-diethylaminophenol into 50 mL of anhydrous toluene, and heat to reflux for 7 hours. After the reaction was completed, cool to room temperature, filter, wash the filter cake with ether, and dry the solid under vacuum to obtain 3.28 g of beige solid 7-(diethylamino)-4-hydroxychromen-2-one with a yield of 28.2%.
Embodiment 2
[0026] Example 2. Synthesis of 7-(N,N-diethylamino)-4-chlorocoumarin-3-formaldehyde
[0027]Under nitrogen protection, 2.8 mL of dry redistilled DMF was slowly added dropwise to 2.8 mL of POCl 3 2.33 g (9.99 mmol) of 7-(diethylamino)-4-hydroxychromen-2-one was dissolved in 13.2 ml DMF, dropwise Add it to the above red solution, and continue to stir the mixture at 60°C for 12 hours under the protection of nitrogen; then pour the reaction solution into 100 mL of ice water, adjust the pH to 6 with 20% NaOH solution, a large amount of precipitates precipitate out, filter, filter cake Wash with an appropriate amount of deionized water three times, and dry the solid in vacuum to obtain 1.84 g of 7-(N,N-diethylamino)-4-chlorocoumarin-3-carbaldehyde, with a yield of 66.7%.
Embodiment 3
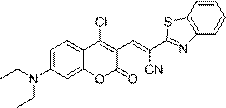
[0028] Embodiment 3. synthesis ( E )-2-(Benzo[d]thiazol-2-yl)-3-(4-chloro-7-(diethylamino)-2-oxo-2H-chromen-3-yl)acrylonitrile
[0029] Add 100 mg (357.50 μM) of 7-(N,N-diethylamino)-4-chlorocoumarin-3-carbaldehyde and 62.3 mg (357.50 μM) of benzothiazole-2-acetonitrile into 7 mL of anhydrous In ethanol, react at room temperature overnight, after the reaction is completed, the reaction solution is filtered, and the resulting solid is vacuum-dried to obtain ( E )-2-(Benzo[d]thiazol-2-yl)-3-(4-chloro-7-(diethylamino)-2-oxo-2H-chromen-3-yl)acrylonitrile 99.9 mg, yield 63.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com