A kind of 1,4-naphthoquinone derivatives and its preparation method and application
A technology of derivatives, naphthoquinones, applied in the fields of new compound synthesis and pharmaceutical application, can solve the problem of no sulfonamide group side chain, etc., achieves simple operation, good anti-human colon cancer cell activity, cheap and easily available raw materials and reagents Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of 1,4-naphthoquinone derivatives according to claim 1, the steps are as follows:
[0042] Diamine mono(Boc) 2 O is protected to obtain III, reacted with sulfonyl chloride to obtain IV, removed the Boc protecting group under acidic conditions to obtain V, reacted with 1,4-naphthoquinone and its derivatives to obtain a class of 1,4-naphthoquinone derivatives, and the reaction formula is
[0043]
[0044] Specifically related to the synthesis of compound 1.
[0045]
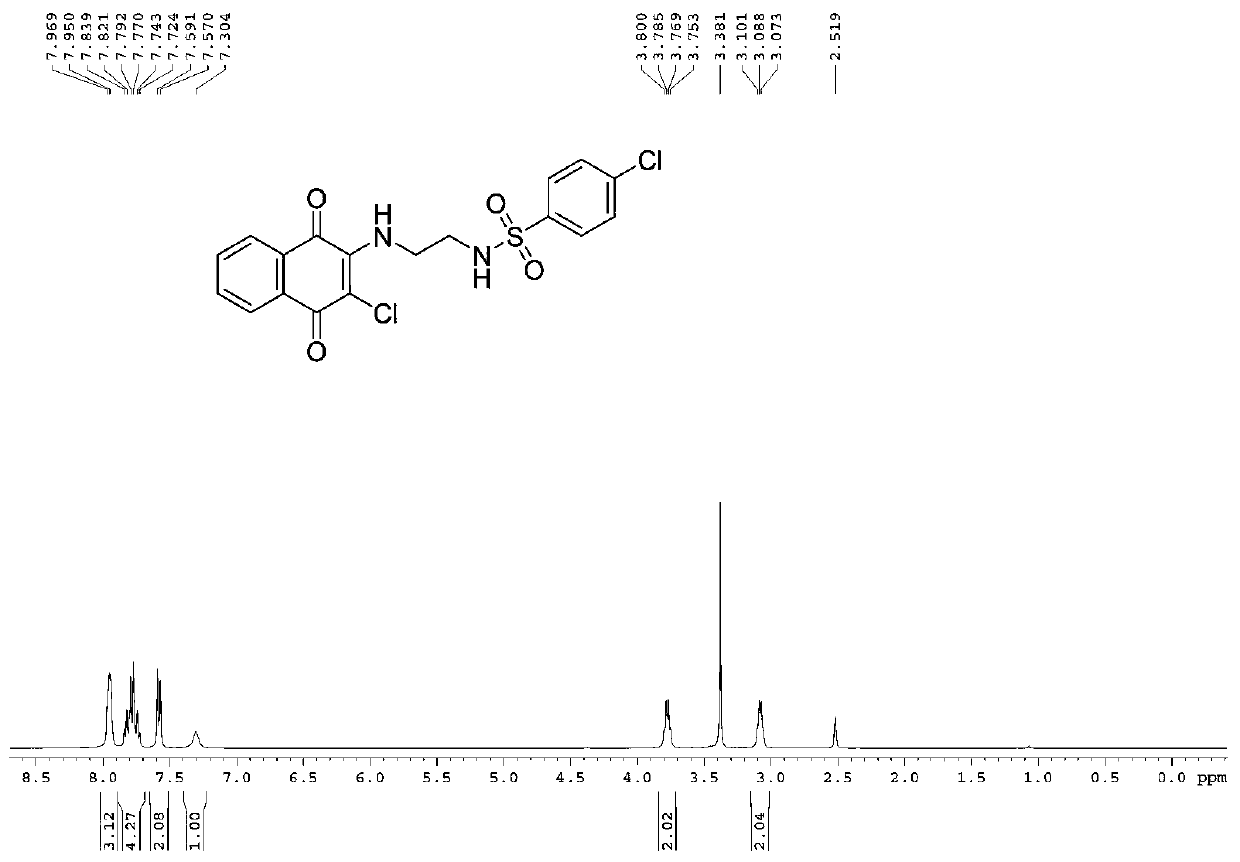
[0046] Will (Boc) 2 O (3.6g, 0.1eq.) was dissolved in anhydrous dichloromethane (50mL), and (Boc) 2 O solution was added dropwise to anhydrous dichloromethane solution of ethylenediamine (0.2mol / L, 1.0eq.) under the protection of argon. The reaction mixture was stirred at 0 °C for 1 h, then at room temperature for 10 h. Saturated NaCl aqueous solution (30mL) was washed, and the organic layer was washed with NaCl 2 SO 4 Drying, filtration and evaporation of the solvent gave th...
Embodiment 2
[0051]Synthesis of compound 2.
[0052]
[0053] The synthesis method of Example 2 is the same as that of Compound 1 above.
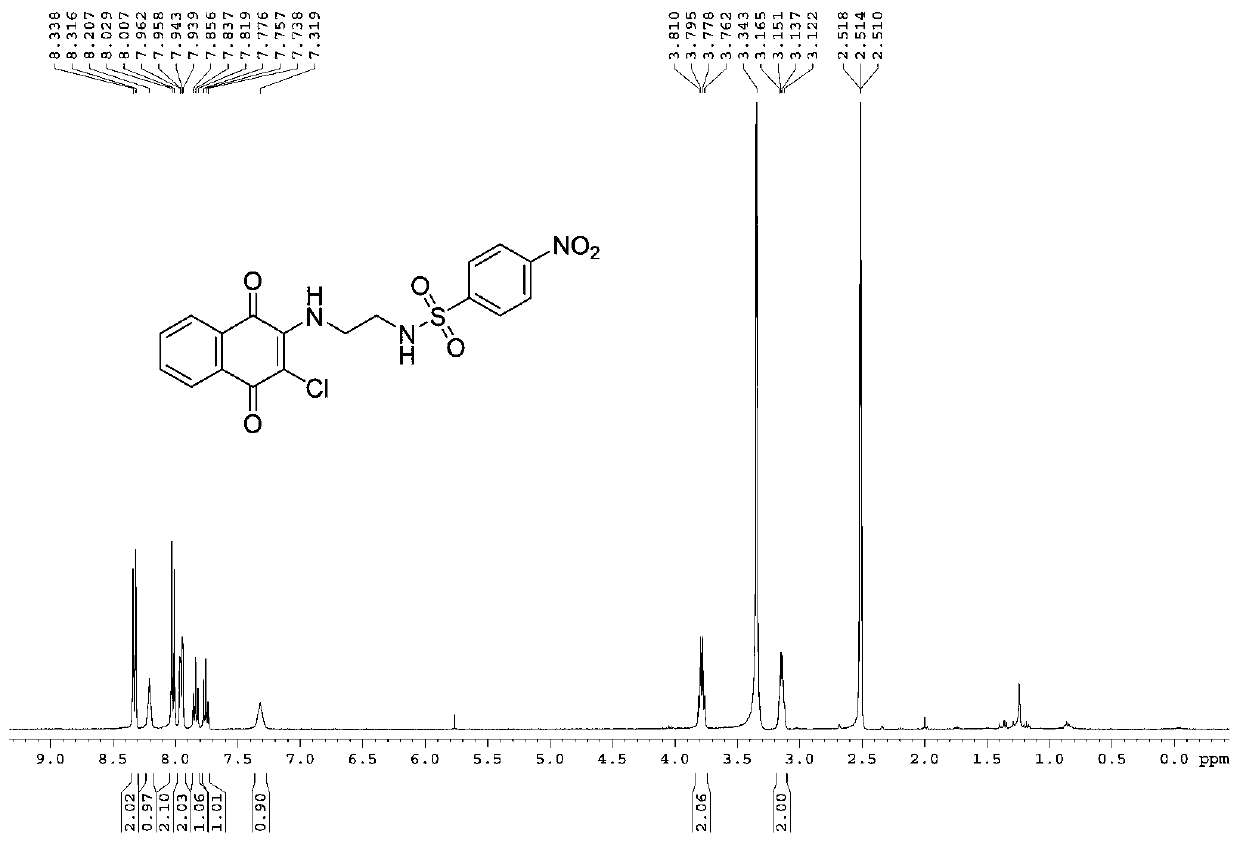
[0054] Yield: 89%; Structural parameters: 1 H NMR (400MHz, DMSO-d 6 )δ7.95(d,J=7.6Hz,3H),7.83–7.72(m,4H),7.58(d,J=8.4Hz,2H),7.30(s,1H),3.80–3.75(m,2H ),3.10–3.07(m,2H); 13 CNMR (100MHz, DMSO-d 6 )δ180.3,175.9,145.5,139.6,137.8,135.3,133.2,132.2,130.3,129.7,128.8,126.9,126.3,43.5.HRMS(ESI-TOF)m / z calcd.for C 18 h 14 N 2 o 4 SCl 2 Na + [(M+Na) + ]:446.9932, found 446.9944.
Embodiment 3
[0056] Synthesis of compound 3.
[0057]
[0058] The synthesis method of Example 3 is the same as the synthesis method of Compound 1 above.
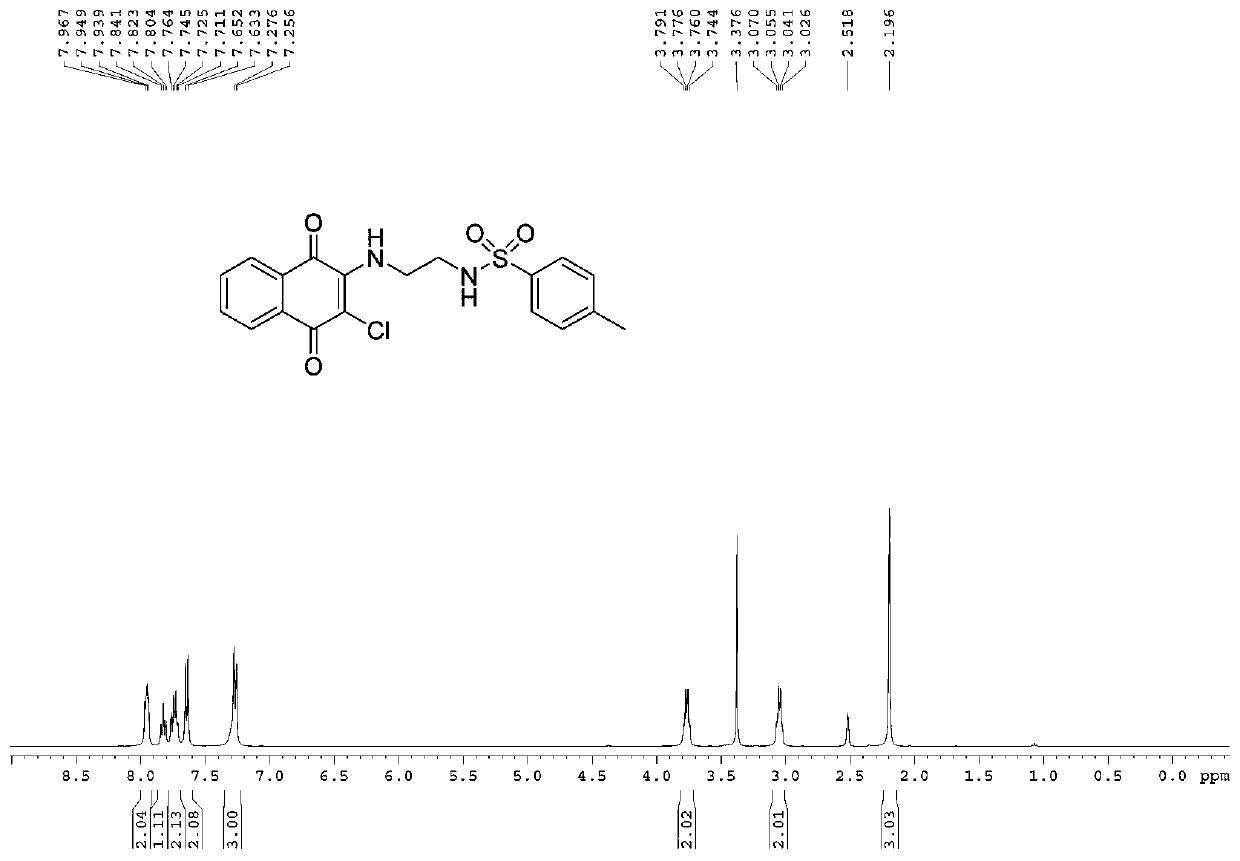
[0059] Yield: 94%; Structural parameters: 1 H NMR (400MHz, DMSO-d 6 )δ7.94(t, J=7.2Hz, 2H), 7.82(t, J=8.4Hz, 1H), 7.76–7.71(m, 2H), 7.64(d, J=7.6Hz, 2H), 7.26( d,J=8.0Hz,3H),3.79–3.74(m,2H),3.07–3.02(m,2H),2.19(s,3H); 13 C NMR (100MHz, DMSO-d 6 )δ180.2, 175.8, 145.4, 143.0, 137.8, 135.3, 133.2, 132.2, 130.3, 130.0, 1126.9, 126.9, 126.2, 43.5, 21.2. 19 h 17 N 2 o 4 SClNa + [(M+Na) + ]:427.0480, found 427.0490.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com