Tetrapeptide with function of resisting Abeta protein aggregation and application of tetrapeptide as well as gene for encoding tetrapeptide
A protein aggregation and residue technology, applied in the field of peptides, can solve problems such as lack of specificity, and achieve the effects of improving memory, delaying the disease process, and major social and economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
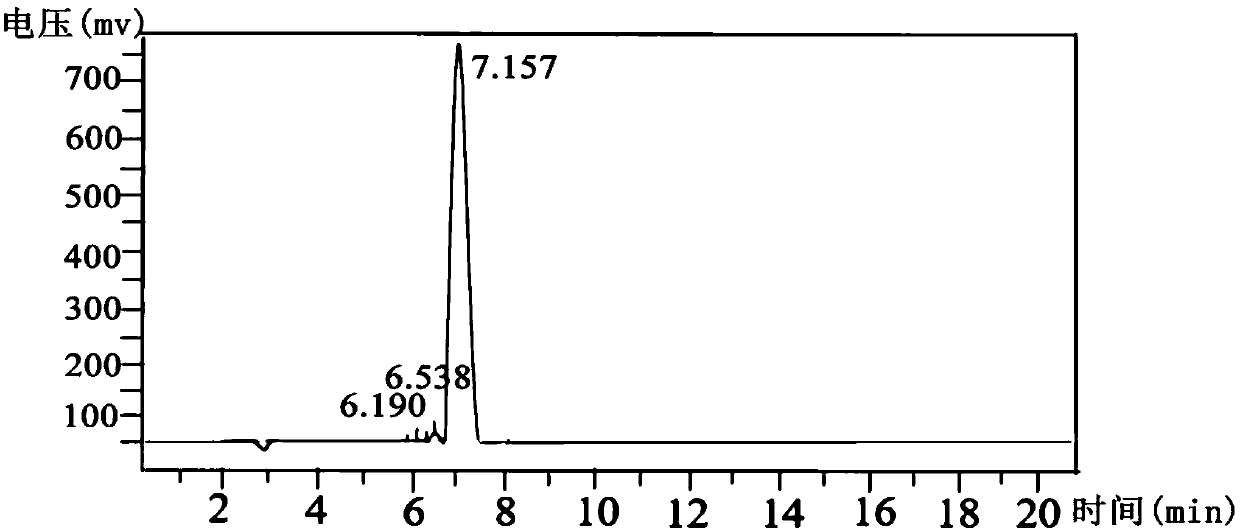
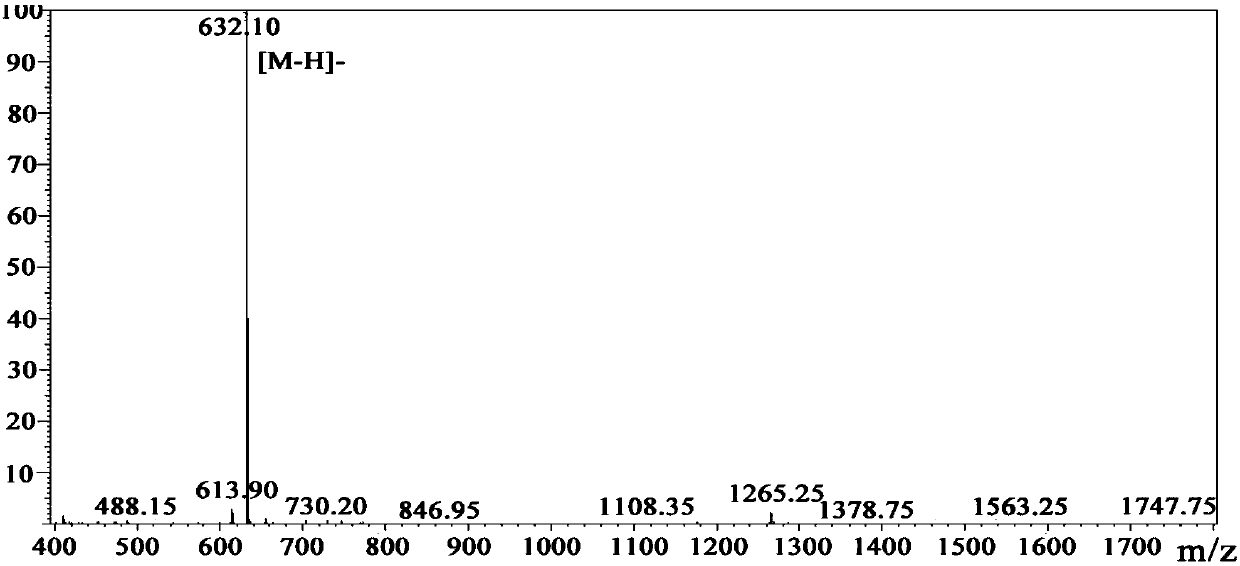
[0040] Synthesis of Peptide WW-4 by Solid Phase Synthesis of Peptide
[0041] 1. Resin selection
[0042] (1) Using the standard Fomc protocol, initially select 0.0125mmol 2-chlorotrityl chlorideresin resin (Tianjin Nankai Synthetic Technology Co., Ltd.), and add 0.3mol For the first Fmoc protection of amino acids, DCC and 5% (mass fraction) DMAP were added to the reactor to shake the reaction, and the resin was washed with methylpyrrolidone (NMP) to remove excess protected amino acids.
[0043] (2) Using the standard Fomc scheme, initially select 0.0125mmol Wang resin, according to the sequence characteristics from the C-terminal to the N-terminal of the amino acid sequence Trp-Asp-Gln-Trp, add 0.3mol of the first Fmoc protected amino acid, DCC and 5% (mass fraction) DMAP is added to the reactor to shake and react, and the resin is washed with NMP to remove excess protected amino acids.
[0044] The coupling ratios of the two resins are: 95.07% for 2-chlororityl chloride re...
Embodiment 2
[0050] Anti-Aβ42 Protein Aggregation Activity Experiment of Synthetic Polypeptide WW-4 in Vitro
[0051] 1. Experimental method
[0052] Preparation of culture medium: High glucose medium (DMEM), fetal bovine serum (FBS), L-glutamine were prepared according to the mass ratio of 8.75:1:0.25 respectively; 1wt% double antibodies (penicillin and streptomycin) were added at the same time , 0.1 wt% Hygromycin B and 0.05 wt% Blasticidin S antibiotics.
[0053] Preparation of 0.05mM and 0.5mM polypeptide (WW-4) solutions: Weigh 11.4mg of polypeptide WW-4, dissolve it with 10mL of medium, pass through a 0.22μm filter head, the concentration of the mother solution is 1mM, and then use the medium to dissolve The mother liquor was diluted to the concentration required for the experiment.
[0054] Preparation of 1 mg / mL tetracycline solution: Weigh 10 mg of tetracycline, prepare with 10 mL of PBS buffer, pass through a 0.22 μm filter head, and store at -20°C in the dark for future use. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com