Preparation method of azilsartan
A compound and reaction technology, applied in the field of drug synthesis, can solve the problems of unfavorable industrial production, long process route, and many by-products, and achieve the effects of shortening post-processing time, avoiding by-products, and improving reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
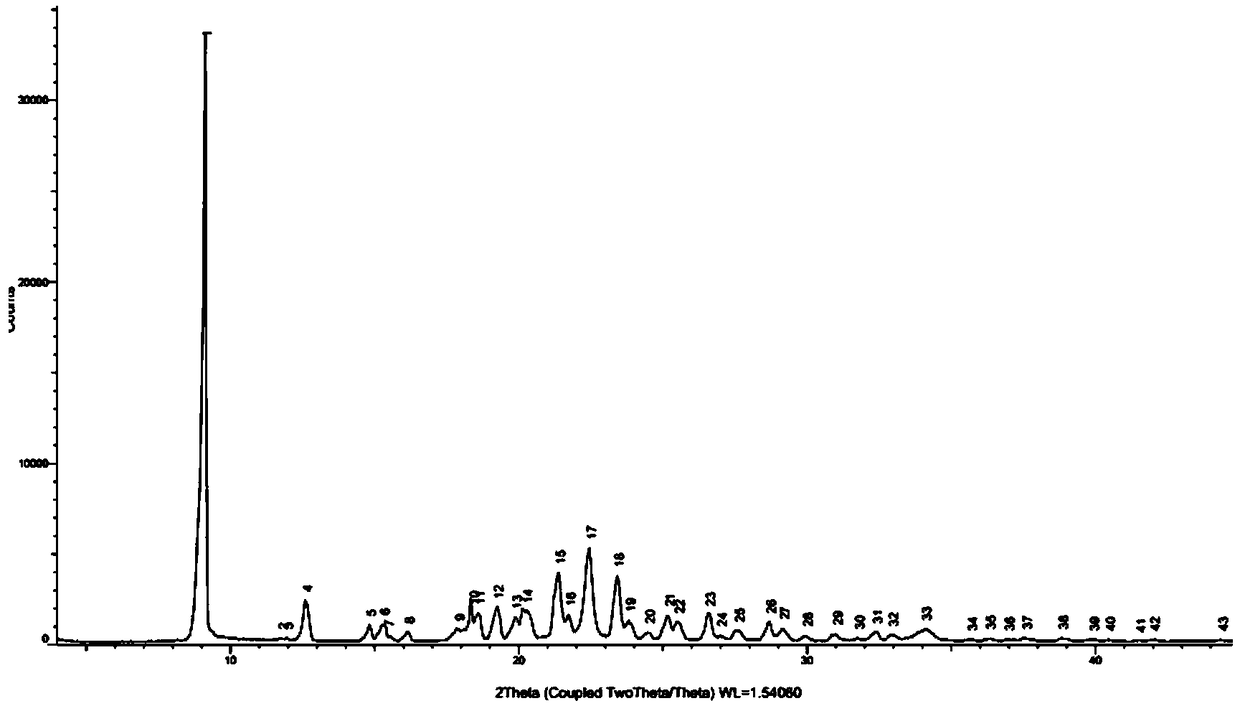
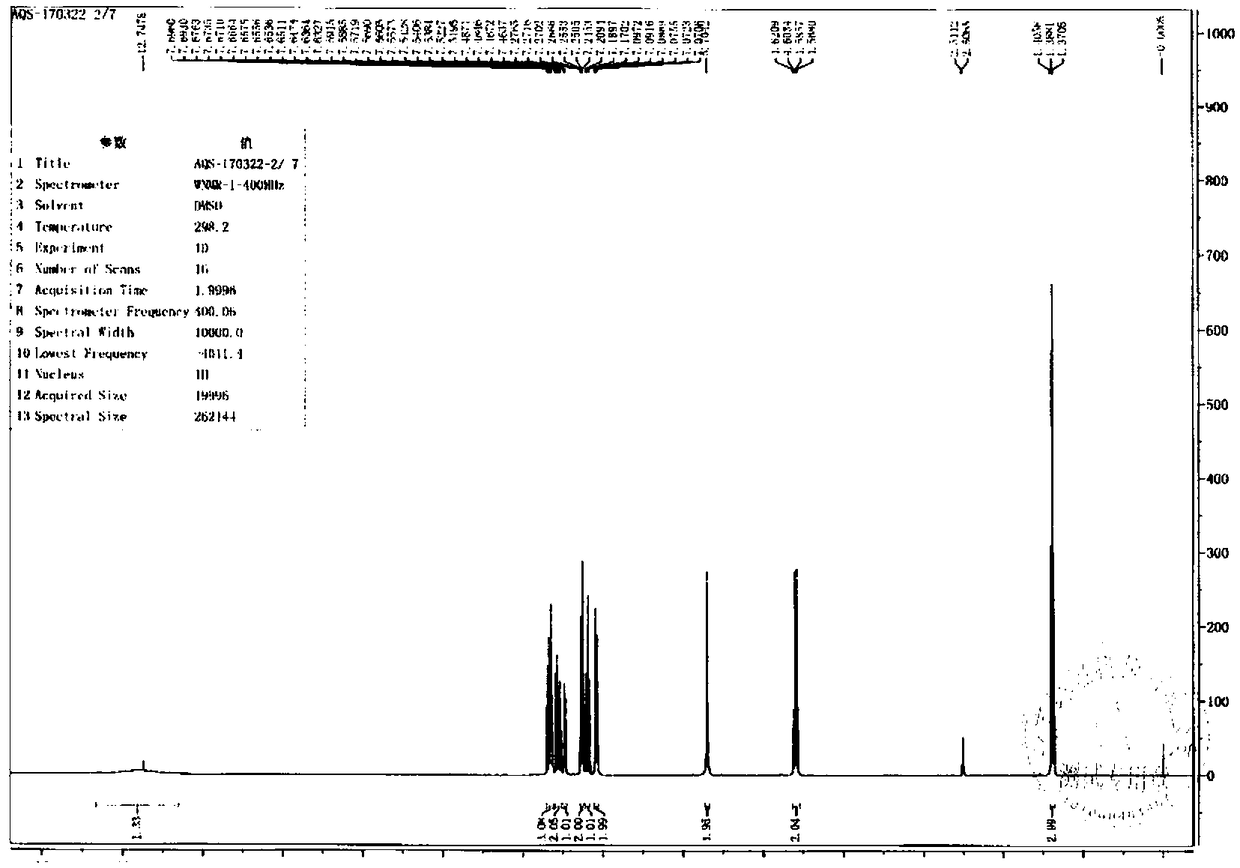
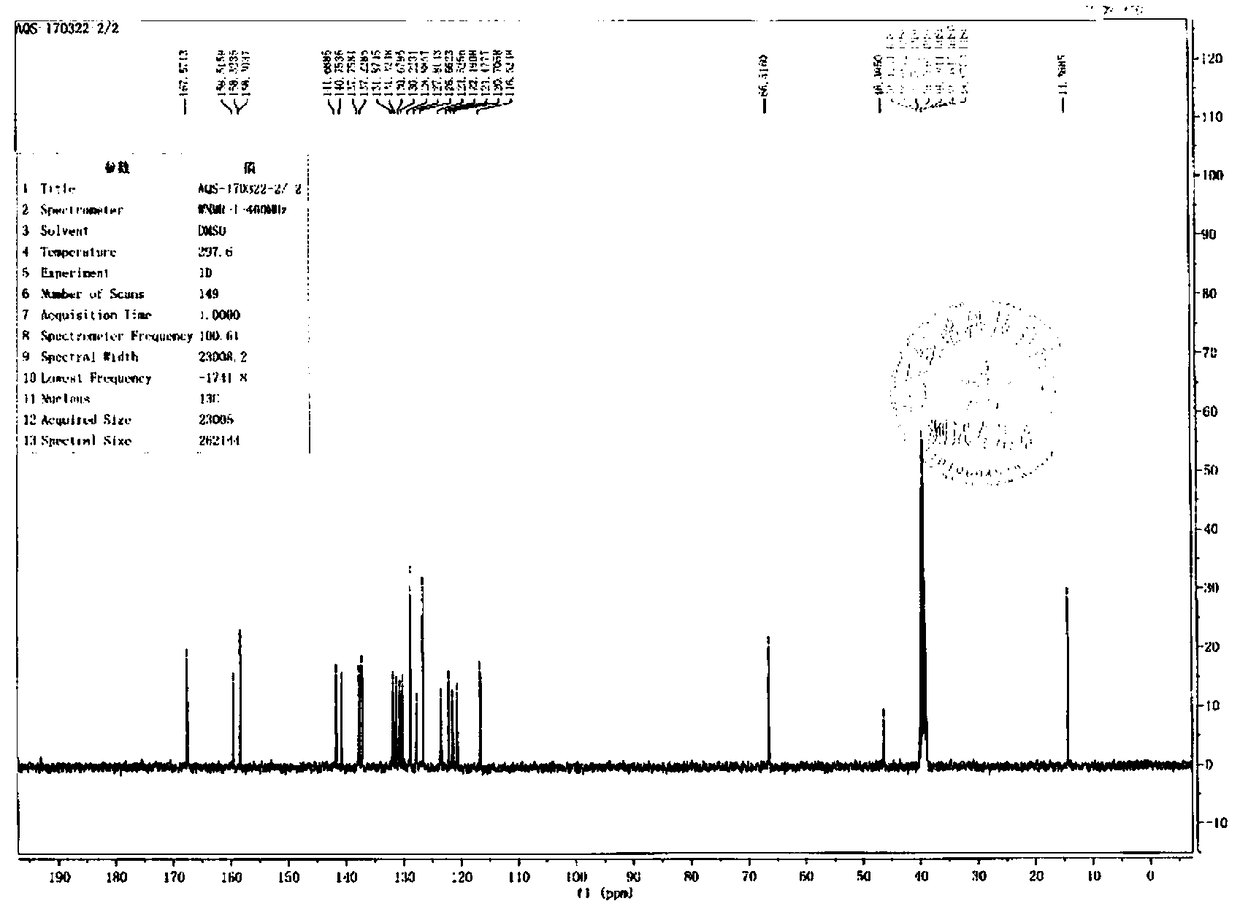
Image
Examples
Embodiment 1
[0046] Add 19.76kg (235.0mol) of sodium bicarbonate and 12.24kg (176.3mol) of hydroxylamine hydrochloride into 110.kg of DMSO, stir and raise the temperature to 50-60°C, stir for 1 hour, and add 25.04kg (58.8mol) of the compound to the above reaction solution 5. Raise the temperature to 75-85°C, keep stirring and react for 7-10 hours. After the reaction, the reaction solution was added to 200.16kg of purified water, stirred at 20-30°C for 1 hour, centrifuged, and the filter cake was rinsed with 12.24kg of purified water and dried to obtain 22.23kg of compound 6 with a yield of 82.5%.
[0047] Add 22.10kg (48.2mol) of compound 6 and 12.50kg (77.1mol) of carbonyldiimidazole into 146.41kg of dichloromethane, stir to dissolve, control the temperature at 25-30°C, add 9.73kg (100.9mol) of triethylenediamine and 87.85kg of dichloromethane mixed solution, reacted at 25~30°C for 2~3h, after the reaction was complete, adjusted pH=3 with 196.52kg of 1N hydrochloric acid, discarded the aq...
Embodiment 2
[0051] Add 29.43kg (350.3mol) of sodium bicarbonate and 20.42kg (293.8mol) of hydroxylamine hydrochloride into 110.kg of DMSO, stir and raise the temperature to 50-60°C, stir for 1 hour, and add 25.00kg (58.8mol) of the compound to the above reaction solution 5. Raise the temperature to 75-85°C, keep stirring and react for 7-10 hours. After the reaction, the reaction solution was added to 200.16kg of purified water, stirred at 20-30°C for 1 hour, centrifuged, and the filter cake was washed with 12.24kg of purified water and dried to obtain 21.84kg of compound 6 with a yield of 81.1%.
[0052] Add 21.80kg (47.5mol) of compound 6 and 26.98kg (166.4mol) of carbonyldiimidazole into 146.41kg of dichloromethane, stir to dissolve, control the temperature at 25-30°C, add 21.33kg (190.2mol) of triethylenediamine and 87.85kg of dichloromethane mixed solution, reacted at 25-30°C for 2-3 hours, after the reaction was complete, adjusted pH=3 with 410.61kg of 1N hydrochloric acid, discarded t...
Embodiment 3
[0056] Add 14.81kg (176.3mol) of sodium bicarbonate and 8.17kg (117.5mol) of hydroxylamine hydrochloride into 110.kg of DMSO, stir and raise the temperature to 50-60°C, stir for 1 hour, and add 25.00kg (58.8mol) of the compound to the above reaction solution 5. Raise the temperature to 75-85°C, keep stirring and react for 7-10 hours. After the reaction, the reaction solution was added to 200.16kg of purified water, stirred at 20-30°C for 1 hour, centrifuged, and the filter cake was rinsed with 12.25kg of purified water and dried to obtain 19.93kg of compound 6 with a yield of 74.0%.
[0057] Add 19.80kg (43.2mol) of compound 6 and 8.40kg (51.8mol) of carbonyldiimidazole into 146.41kg of dichloromethane, stir to dissolve, control the temperature at 25-30°C, add 7.27kg (64.8mol) and 87.85kg of dichloromethane Methane mixture, react at 25-30°C for 2-3 hours, after the reaction is complete, use 176.1kg 1N hydrochloric acid to adjust pH=3, discard the water phase after liquid separ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com