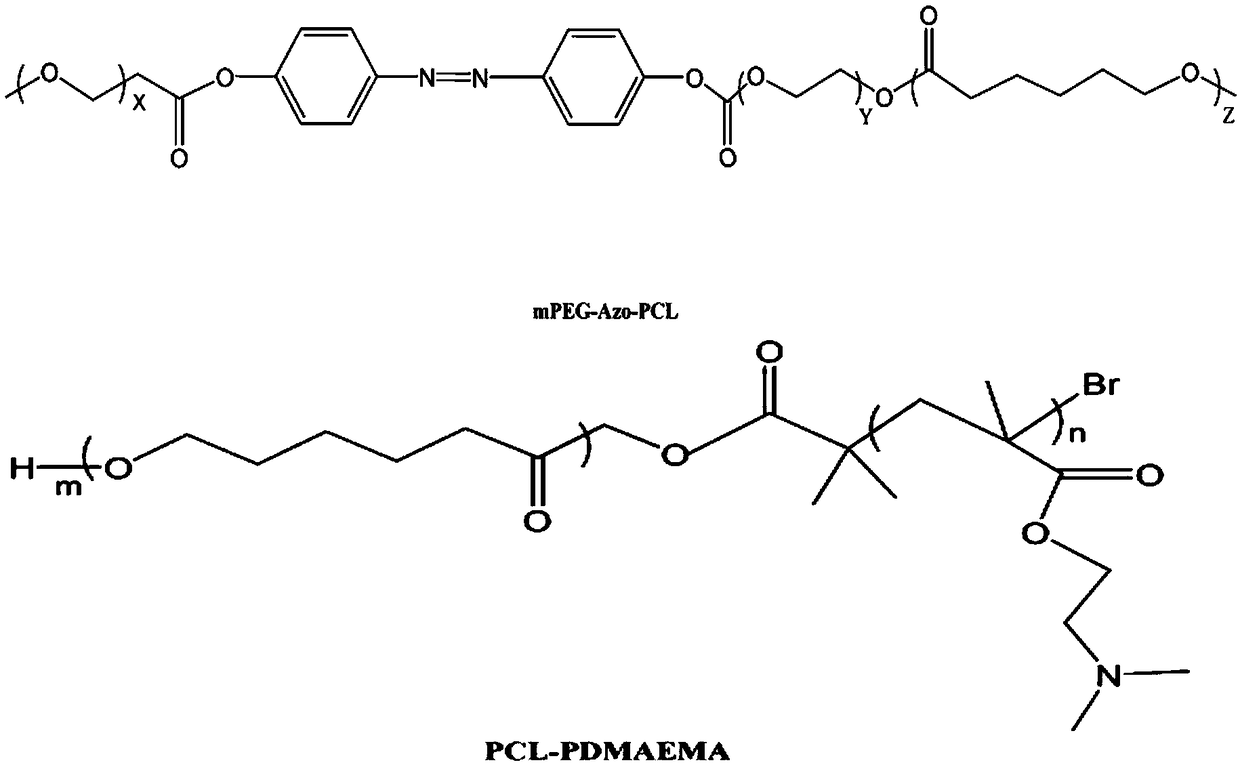
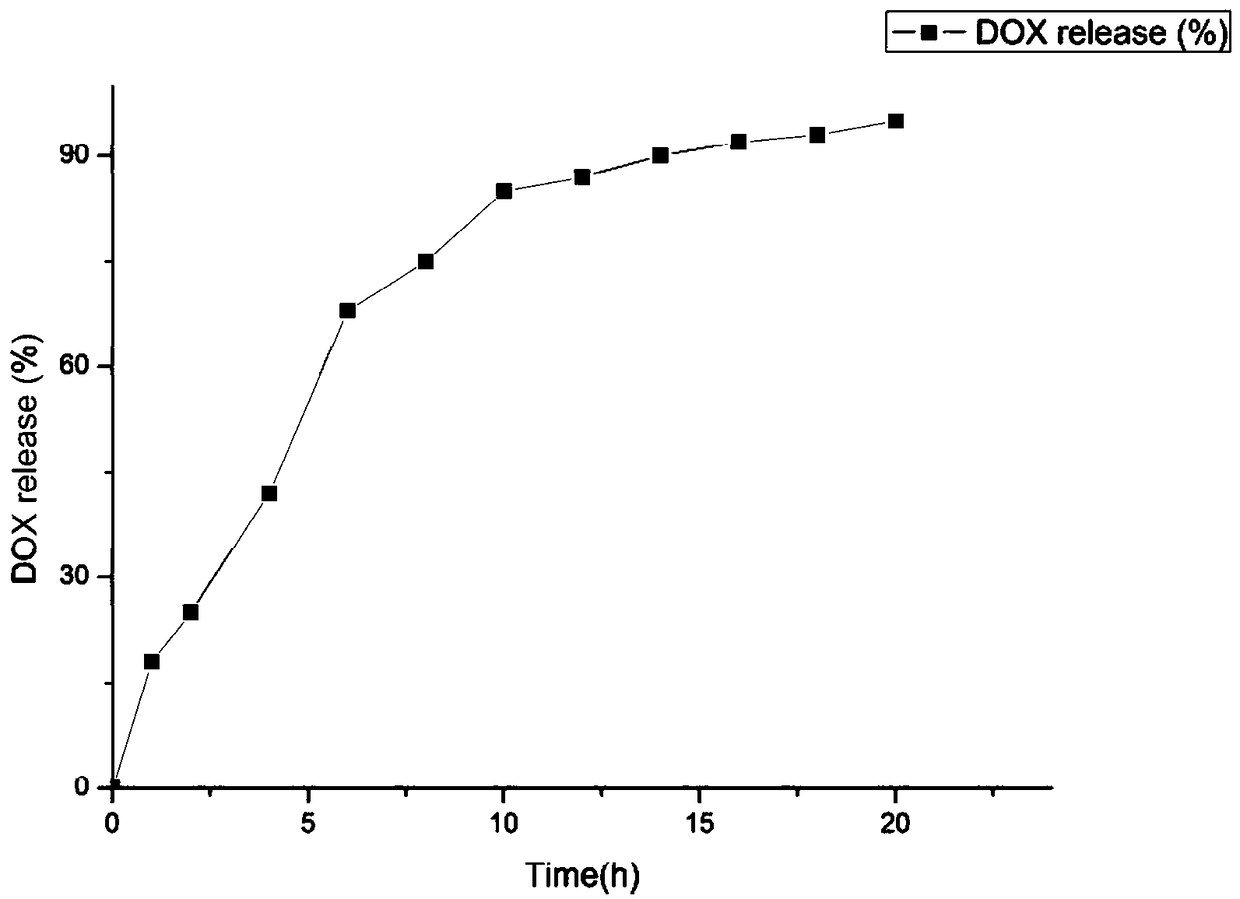
Mixed micelle with hypoxia and pH double responsiveness and preparation method of mixed micelle
A mixed micelle, responsive technology, applied in the fields of polymer materials and biomedical engineering, can solve the problem of insufficient physiological system, and achieve the effect of broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Weigh 1.12g p-hydroxyazobenzene, 0.65g N,N'-dicyclohexylcarbodiimide, 0.13g 4-dimethylaminopyridine and dissolve in dichloromethane and dimethylformamide 1:1 In the mixed solvent, weigh 2.004g of carboxylated monomethoxypolyethylene glycol and add it dropwise in the solution, the reaction is carried out under the protection of nitrogen, the reaction temperature is room temperature, and the reaction time is 24 hours. By-products were removed by vacuum filtration, and the collected product was precipitated in ice-cold diethyl ether. The product was transferred to a reaction flask, and cyclohexyl lactone and a catalyst, stannous isooctanoate, were added. Under an ice-water bath, vacuum was used to remove oxygen and nitrogen gas was introduced. The ice-water bath was removed, the reaction temperature was adjusted to 150° C., and the reaction time was 24 hours. After the product was cooled to room temperature, diethyl ether was used as a precipitant to precipitate the p...
Embodiment 2
[0026] (1) Weigh 0.93g p-hydroxyazobenzene, 0.54g N,N'-dicyclohexylcarbodiimide, 0.09g 4-dimethylaminopyridine and dissolve in dichloromethane and dimethylformamide 1:2 In the mixed solvent, weigh 1.84g carboxylated monomethoxypolyethylene glycol and add it dropwise to the solution. The reaction is carried out under the protection of nitrogen, the reaction temperature is room temperature, and the reaction time is 16 hours. Remove by filtration, collect the product and precipitate in frozen diethyl ether, transfer the product to a reaction flask, add 4.8g cyclocaprolactone, 0.47g catalyst stannous isooctanoate, under ice-water bath, vacuum to remove oxygen and pass nitrogen. The ice-water bath was removed, the reaction temperature was adjusted to 100°C, and the reaction time was 24 hours. After the product was cooled to room temperature, it was precipitated in the precipitating agent n-hexane to obtain the polymer PEG-Azo-PCL and dried in vacuum.
[0027](2) Weigh 5.6g of hydro...
Embodiment 3
[0030] (1) Weigh 1.58g of p-hydroxyazobenzene, 0.72g of N,N'-dicyclohexylcarbodiimide, and 0.21g of 4-dimethylaminopyridine and dissolve them in dichloromethane and dimethylformamide at a ratio of 1:2 2.14g carboxylated monomethoxypolyethylene glycol was added dropwise in the solution, the reaction was carried out under the protection of nitrogen, the reaction temperature was room temperature, and the reaction time was 20 hours, and the by-product was vacuum filtered Remove, collect the product and precipitate it in frozen ether, transfer the product to a reaction flask, add 6.4g cyclocaprolactone, 0.46g catalyst stannous isooctanoate, under ice-water bath, vacuum to remove oxygen and pass nitrogen. The ice-water bath was removed, the reaction temperature was adjusted to 150°C, and the reaction time was 28 hours. After the product was cooled to room temperature, it was precipitated in the precipitating agent cyclohexane to obtain the polymer PEG-Azo-PCL and dried in vacuum.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com