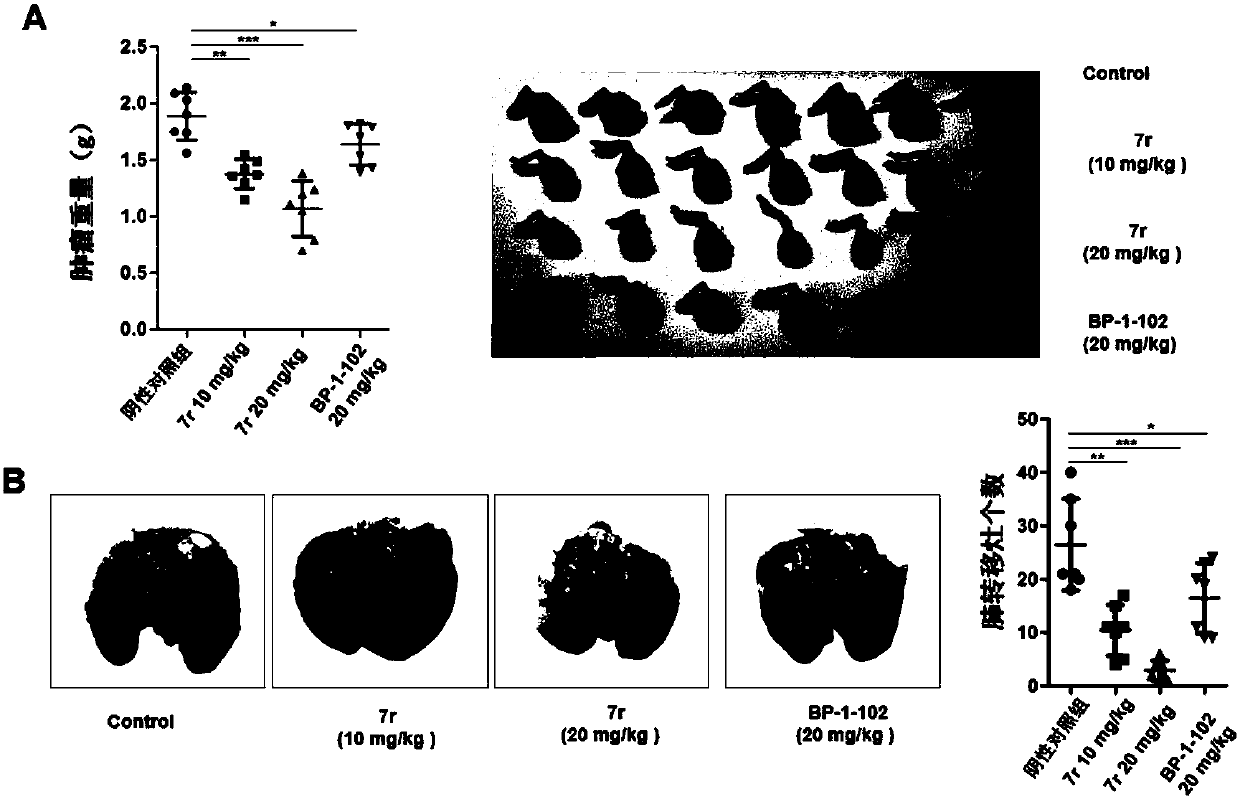
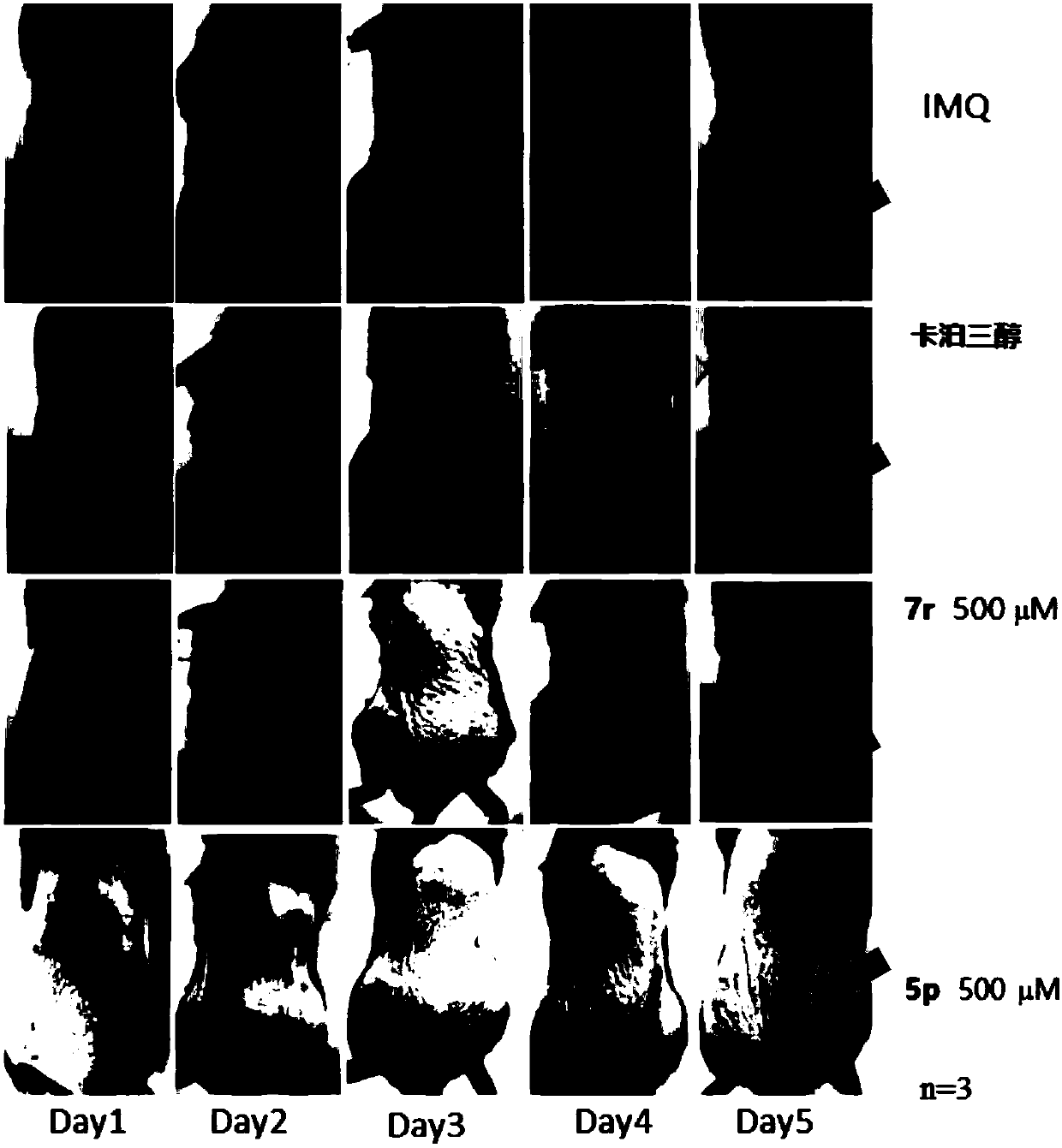
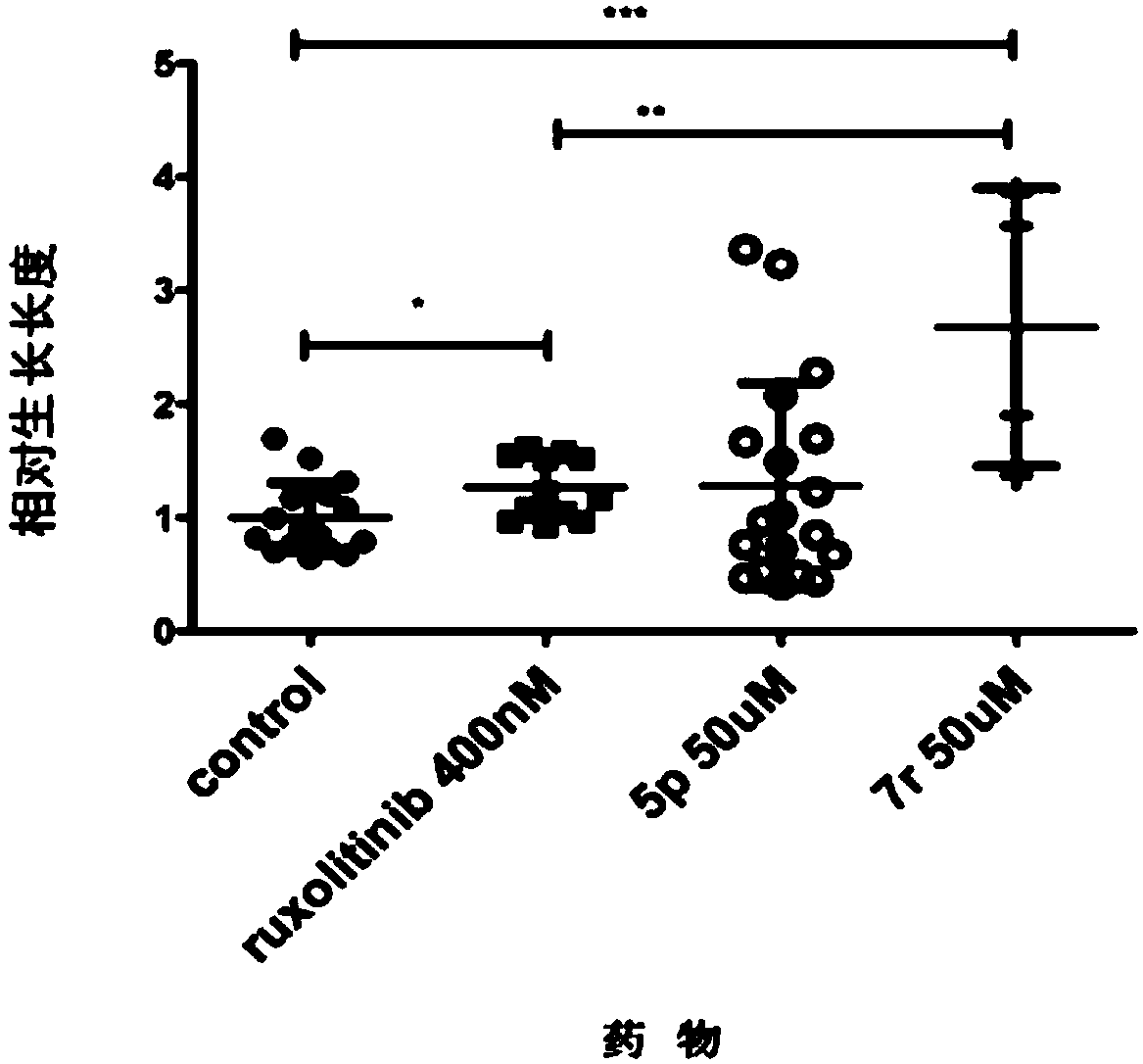
Cycloalkane thiophthene derivative as well as preparation method and medical application thereof
A technology of thiophene derivatives and cycloalkanes, applied in the field of medicine, can solve the problems of unstable metabolism, difficult-to-clinical compounds, toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-14
[0188] Example 1-1 Synthesis of 4-cyano-5-(1-methyl-4-pyrazole)-carboxamido-cyclobutyl[2,3]thiophene (1a).
[0189] Target products 1a-1e, 2a-2j are shown in formula 1-1, cyclobutanone (or other cycloalkanones), malononitrile, solid sulfur and proline are dissolved in DMF, stirred at 60°C for 10 The reaction was completed in 1 hour; the reaction solution was slowly added dropwise to stirred ice water, and a large amount of crude product containing the intermediate 2-amino-3-cyano-thiophene derivative intermediate was precipitated, and after filtration, drying and purification; the intermediate Body, 1-methylpyrazole-4-carboxylic acid, 2-chloro-1-methylpyridinium iodide and DMAP were dissolved in dichloromethane, triethylamine was added dropwise under stirring and then heated to reflux overnight to obtain the target Products 1a-1e, 2a-2j.
[0190]
[0191] Synthetic steps of formula 1-1 1a-1e, 2a-2j
[0192] Weigh cyclobutanone (210mg, 3.0mmol), malononitrile (218mg, 3.3mm...
Embodiment 1-2
[0193] Example 1-2 Synthesis of 4-cyano-5-(1-methyl-4-pyrazole)-carboxamido-cyclopentyl[2,3]thiophene (1b).
[0194] Using the same method for preparing compound 1a, replacing cyclobutanone with cyclopentanone, finally obtained 1b with a yield of 67%. 1 HNMR(500MHz,DMSO)δ11.32(s,1H),8.43(s,1H),8.05(s,1H),3.91(s,3H),2.85(t,J=6.5Hz,2H),2.75( t,J=6.4Hz,2H),2.40-2.36(m,2H).
Embodiment 1-3
[0195] Example 1-3 Synthesis of 2-(1-methyl-4-pyrazole)-carboxamido-3-cyano-4,5,6,7-tetrahydrobenzothiophene (1c).
[0196] Using the same method for preparing compound 1a, replacing cyclobutanone with cyclohexanone, finally obtained 1c with a yield of 63%. 1 HNMR(500MHz,DMSO)δ11.31(s,1H),8.44(s,1H),8.05(s,1H),3.90(s,3H),2.61-2.60(m,2H),2.51-2.50(m ,2H),1.76-1.75(m,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com