Catalyst for degrading phenol in water as well as preparation method and application thereof
A technology of catalyst and phenol, which is applied in the field of catalyst for degrading phenol in water and its preparation, to achieve the effects of easy recovery, improved decomposition rate and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Fe 3 o 4 / Schwittmann stone catalyst preparation:
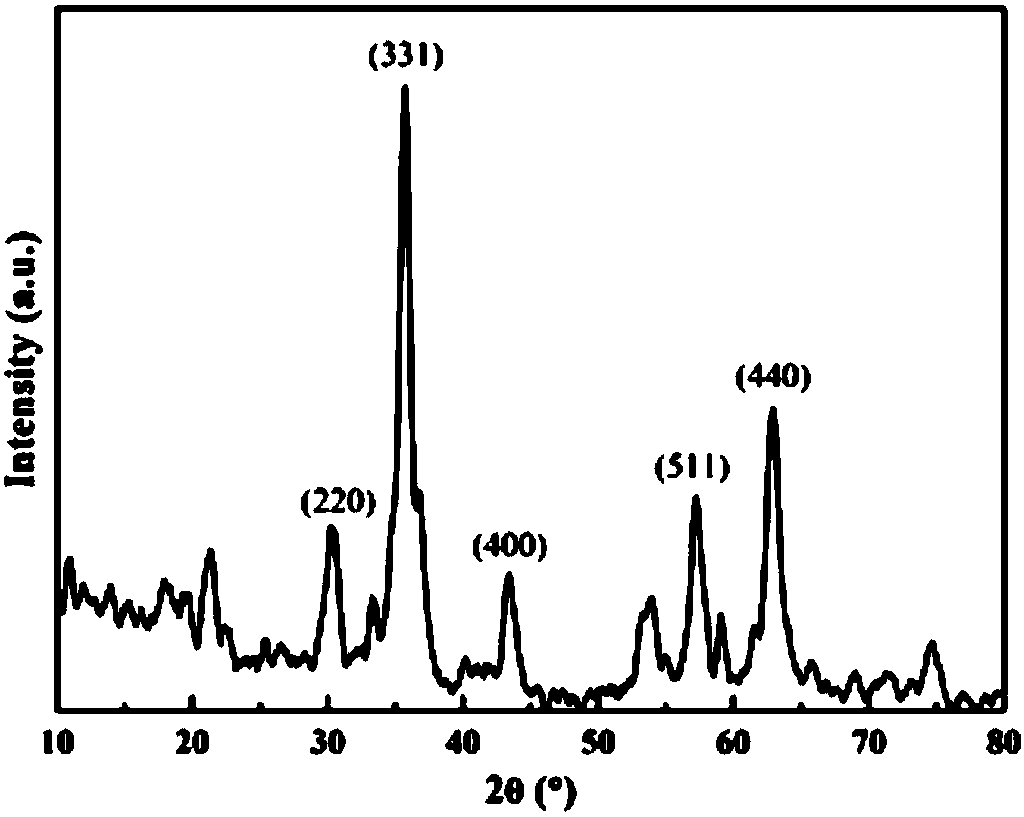
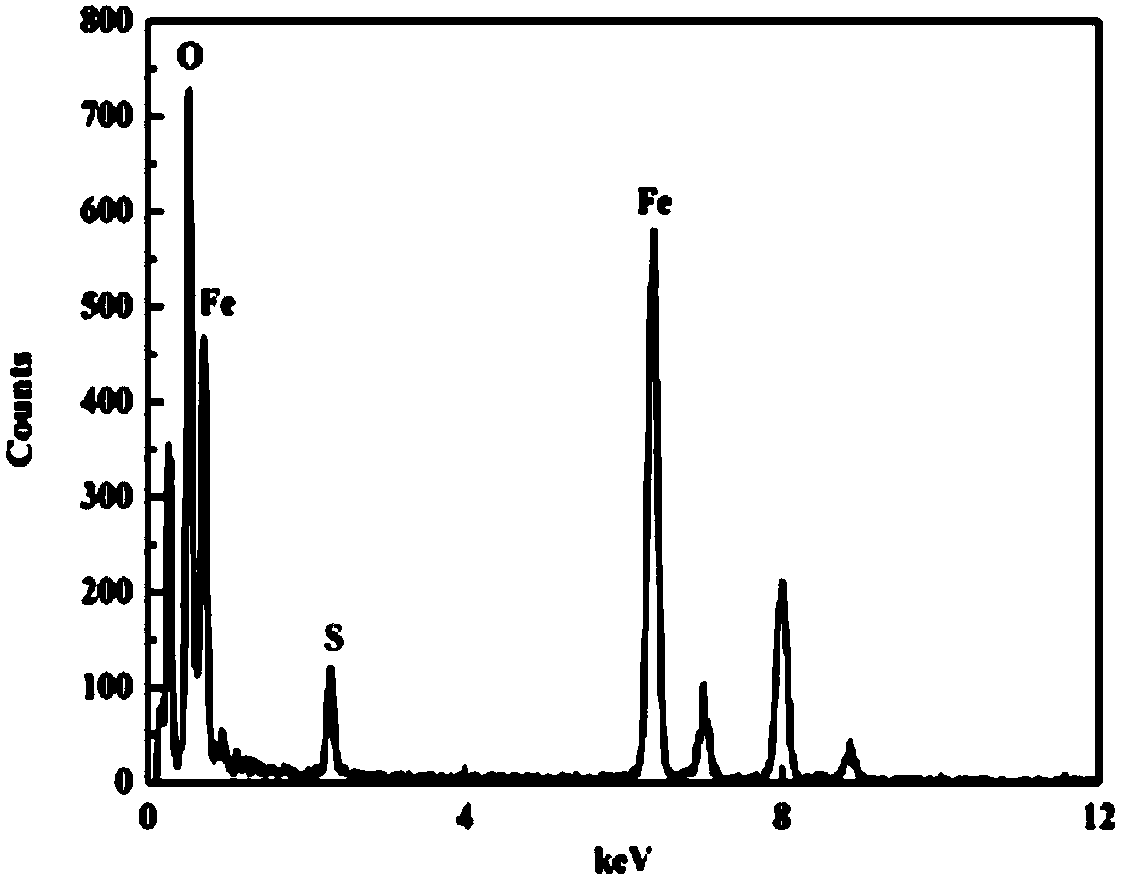
[0030] Add pH = 2.0 diluted H to the Erlenmeyer flask 2 SO 4 solution and 1.0g Fe 3 o 4 Stir evenly, keep the total volume of the reaction system at 500mL, place on a 180r / min reciprocating shaker at 28°C, and shake for 2 hours; then add 11.12g FeSO 4 ·7H 2 O and 6mL H 2 o 2 (concentration is 1.11g / mL); put the mixed solution on a reciprocating shaker at 180r / min at 28°, and shake and cultivate for 24 hours; 2 SO 4 The aqueous solution was washed 3 times, and then washed 3 times with ultrapure water; the samples were preserved after vacuum freeze-drying. From figure 1 It can be seen that the catalyst is magnetic and is easily attracted by a magnet. figure 2 It can be seen that Fe 3 o 4 / Schwittmannite Catalyst in the presence of Fe 3 o 4 , good crystallinity. From image 3 See, Fe 3 o 4 / Schwittmannite catalyst elements include Fe, S and O. The chemical composition of Schwittmannite is Fe 8 o ...
Embodiment 2
[0032] Diluted H at pH = 1.5 was added to the Erlenmeyer flask 2 SO 4 pure aqueous solution and 0.5g Fe 3 o 4 Stir evenly, keep the total volume of the reaction system at 500mL, place on a 200r / min reciprocating shaker at 30°C, and shake for 1 hour; then add 22.2g FeSO 4 ·7H 2 O and 10mL H 2 o 2 (concentration is 1.11g / mL); place the mixed solution on a reciprocating shaker at 200r / min at 30°C, and culture it with shaking for 25 hours; 2 SO 4 The aqueous solution was washed 3 times, and then washed 3 times with ultrapure water; the samples were preserved after vacuum freeze-drying. From figure 1 It can be seen that the catalyst is magnetic and is easily attracted by a magnet. figure 2 It can be seen that Fe 3 o 4 / Schwittmannite Catalyst in the presence of Fe 3 o 4 , good crystallinity. From image 3 See, Fe 3 o 4 / Schwittmannite catalyst elements include Fe, S and O. The chemical composition of Schwittmannite is Fe 8 o 8 (OH) 8-2x (SO 4 ) x, where 1≤x...
Embodiment 3
[0034] Diluted H at pH = 2.5 was added to the Erlenmeyer flask 2 SO 4 Pure aqueous solution and 1.5g Fe 3 o 4 Stir evenly, keep the total volume of the reaction system at 500mL, place on a 250r / min reciprocating shaker at 32°C, and shake for 0.5 hours; then add 26.5g FeSO 4 ·7H 2 O and 23.9 mL H 2 o 2 (concentration is 1.11g / mL); place the mixed solution on a reciprocating shaker at 250r / min at 32°C, and culture it with shaking for 20 hours; 2 SO 4 The aqueous solution was washed 3 times, and then washed 3 times with ultrapure water; the samples were preserved after vacuum freeze-drying. from figure 1 It can be seen that the catalyst is magnetic and is easily attracted by a magnet. figure 2 It can be seen that Fe 3 o 4 / Schwittmannite Catalyst in the presence of Fe 3 o 4 , good crystallinity. from image 3 See, Fe 3 o 4 / Schwittmannite catalyst elements include Fe, S and O. The chemical composition of Schwittmannite is Fe 8 o 8 (OH) 8-2x (SO 4 ) x , wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com