Early specific autoantibody panel diagnosis kit for small cell carcinoma of lung
A technology for early diagnosis of small cell carcinoma, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problem of insufficient sensitivity and specificity to meet lung cancer screening, and achieve high diagnostic value, simple operation, sensitivity and The effect of specificity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 preliminary screening experiment:
[0046] Serum collection: The serum of the control group came from healthy people in the physical examination center. All lung cancer cases were diagnosed by histopathology, according to the latest WHO classification of lung cancer and the 6th edition of UICC staging criteria for lung cancer, and met: ①Patients with primary small cell carcinoma of the lung confirmed by histopathology or cytology. ②Have not received any anti-cancer treatment before blood collection. ③ All patients signed the informed consent.
[0047] First, the patient's serum was hybridized with a chip containing 1627 disease-related proteins to obtain specific binding highlights. The chip was scanned by MicroVigene software (Vigene Tech version 2.9.9.2) to obtain the optical density value. The antigenic protein specifically binding to the antibody in the detection serum was analyzed and screened by the statistical software spss17.0.
[0048] The basis fo...
Embodiment 2
[0053] Example 2 Medium screening experiment scheme:
[0054] 1) Serum collection: Serum from patients with benign lung tumors: ① No anti-cancer treatment was received before blood collection. ② All patients signed the informed consent. The inclusion criteria for all lung cancer cases were the same as the initial screening.
[0055] 2) Screening in the protein chip: Print the antigens corresponding to the 20 antibodies that have been screened on the glass slide to make a chip. Add patient serum or control serum to each chip, add rabbit anti-human secondary antibody, scan, and statistically analyze.
[0056] The basis for screening autoantibodies is: on the premise that the specificity is greater than 91%, the sensitivity is above 20%.
[0057] After intermediate screening, 10 autoantibodies were obtained, namely CDC20, CUL5, ELMOD1, ERCC2, FGF1, HOXB6, KRT8, NFE2, POLDIP3, RRAS2.
[0058] Conclusion: In the screening experiment, the above 10 autoantibodies were obtained af...
Embodiment 3
[0059] Embodiment 3 Verification stage experimental scheme:
[0060] 1) Serum collection: The principle of collection is the same as that of the primary screening stage. But there can be no repeat serum.
[0061] 2) Use the ELISA (rapid enzyme-linked immunosorbent assay) method to detect the relative expression level (OD value) of the effective antibody obtained after the intermediate screening. The method is as follows: in a 96-well plate, after incubation overnight with the antibody coated with anti-GST, the corresponding antigenic protein with the GST label is added, and the antigen is captured by the anti-GST and coated in the well plate. Then add experimental serum to incubate, finally add rabbit anti-human secondary antibody, TMB substrate color development, OD450 reading value.
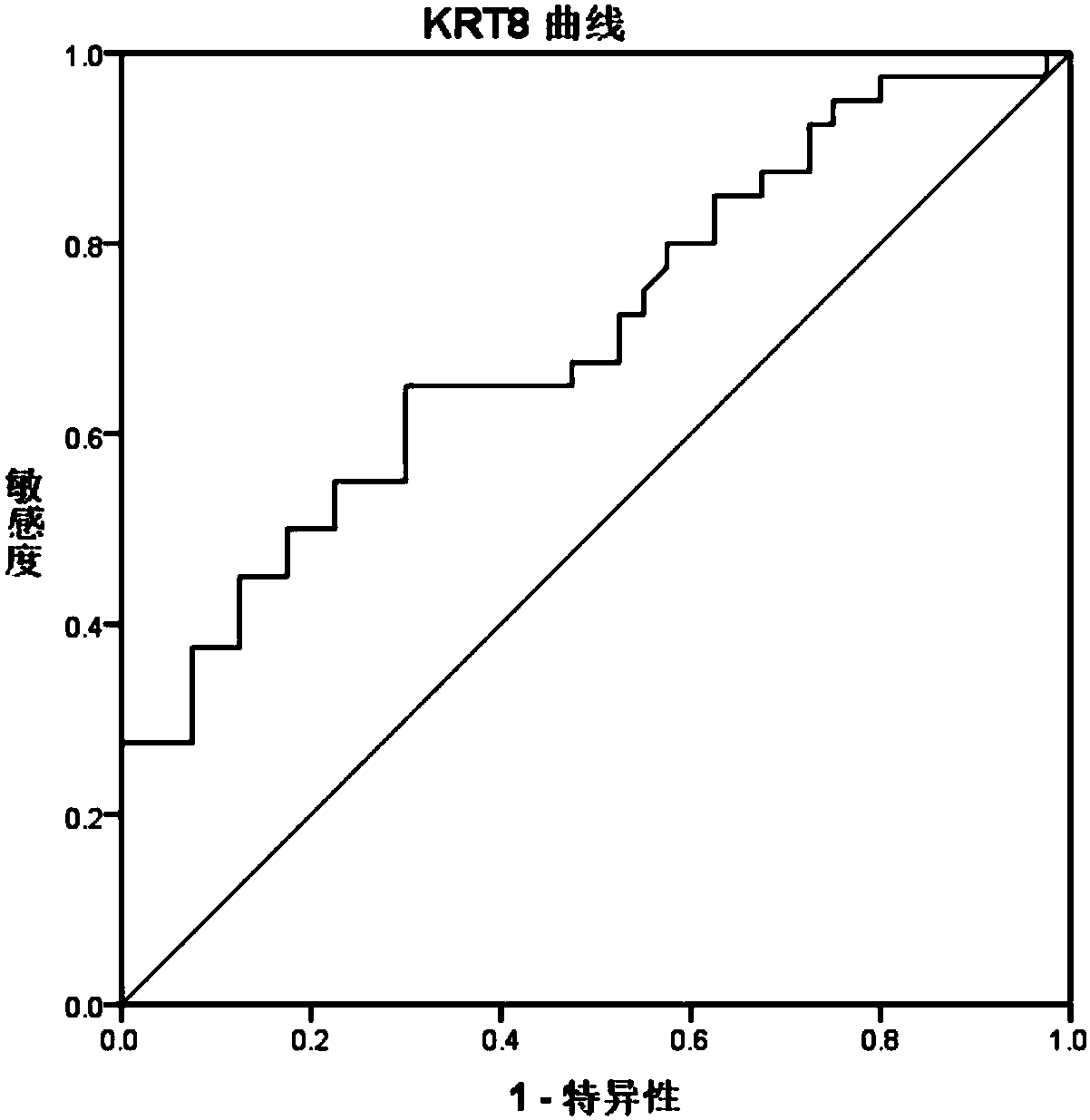
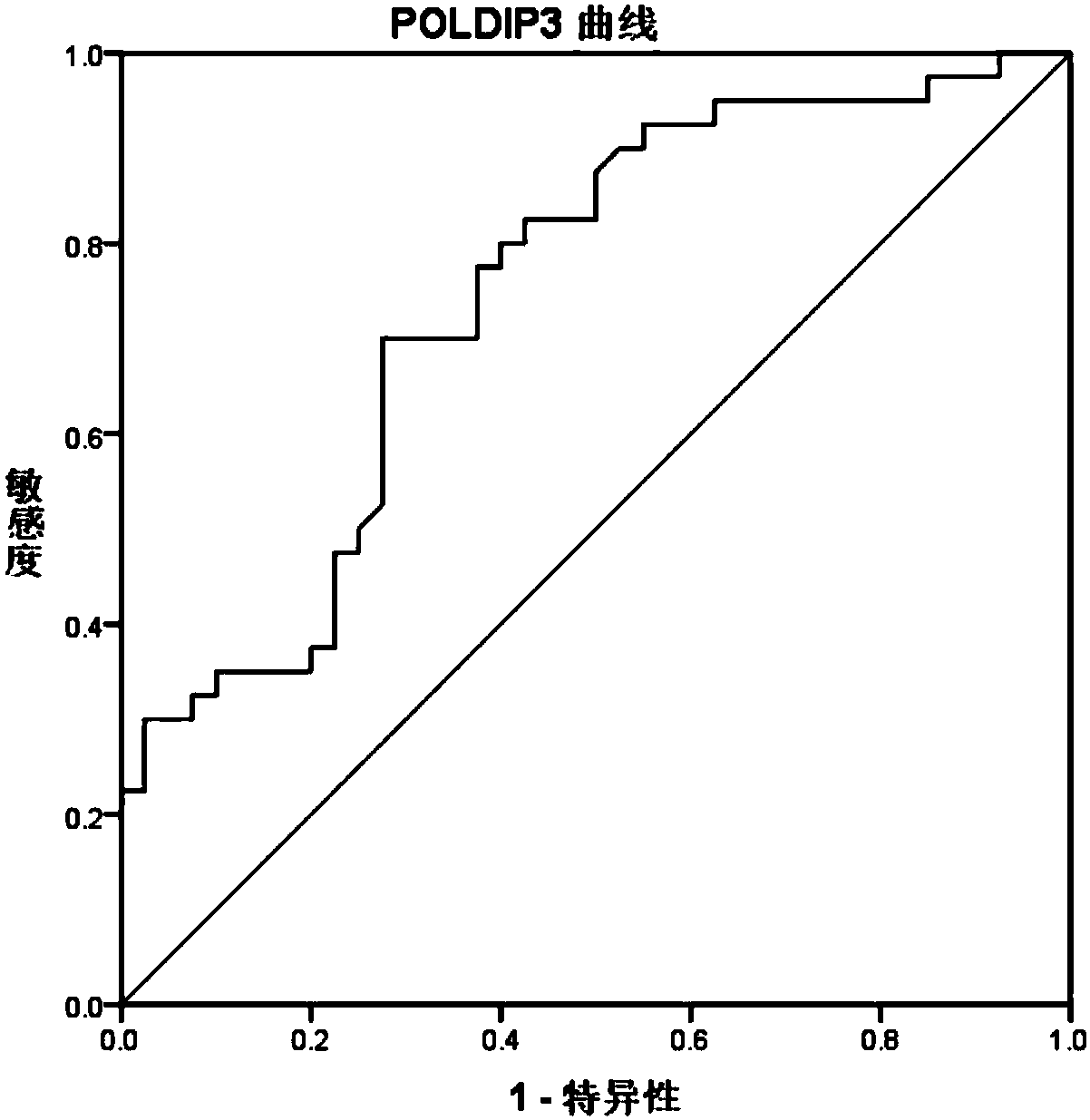
[0062] The basis for screening autoantibodies: under the premise that the specificity is greater than 91%, the sensitivity is above 30%.
[0063] Conclusion: Three specific autoantibodies PO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com