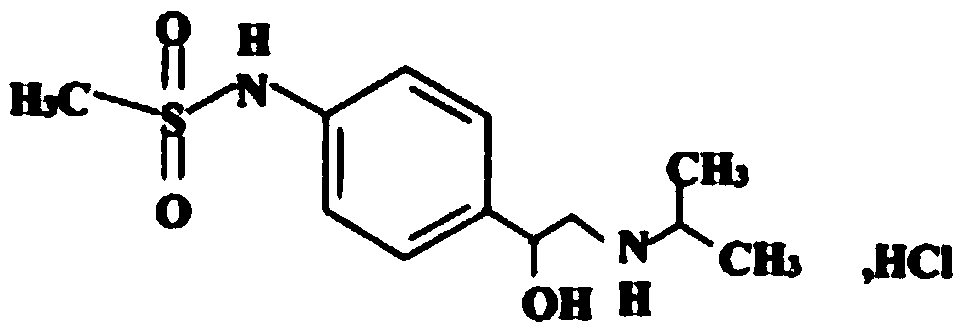
Medicinal composition containing sotalol hydrochloride and preparing method of composition
A technology of sotalol hydrochloride and composition, which is applied in the field of medicinal compositions containing sotalol hydrochloride and its preparation field, can solve potential safety hazards of patients, increase the workload of medical workers, and increase the size of insoluble particles, etc. problems, to achieve the effect of reducing security risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Preparation Process
[0029] The mannitol, sodium bisulfite and sotalol hydrochloride of the prescribed amount are dissolved in water for injection accounting for 80% of the total volume, stirred and mixed evenly; the pH value is adjusted with an appropriate amount of pH value, and the volume is adjusted to the total volume with water for injection. Measure, stir and mix evenly; filter, fill in neutral borosilicate glass injection bottles according to 4ml / bottle, stopper halfway, freeze-dry, and visually inspect to get the finished product.
[0030] Freeze-drying curve:
[0031] 1). Pre-freezing stage: place the filled and semi-stoppered semi-finished product on the partition in the freeze-drying cabinet for pre-freezing, and make the semi-finished product reach the pre-freezing temperature -45°C in the shortest possible time. 150min;
[0032] 2). Sublimation drying stage: the vacuum degree in the drying oven reaches 100mTorr; the main drying temperature i...
Embodiment 2
[0035] prescription
[0036]
[0037] Preparation Process
[0038] The mannitol, sodium bisulfite and sotalol hydrochloride of the prescribed amount are dissolved in water for injection accounting for 80% of the total volume, stirred and mixed evenly; the pH value is adjusted with an appropriate amount of pH value, and the volume is adjusted to the total volume with water for injection. Measure, stir and mix evenly; filter, fill in neutral borosilicate glass injection bottles according to 4ml / bottle, stopper halfway, freeze-dry, and visually inspect to get the finished product.
[0039] Freeze-drying curve:
[0040] 1). Pre-freezing stage: place the filled and semi-stoppered semi-finished product on the partition in the freeze-drying cabinet for pre-freezing, and make the semi-finished product reach the pre-freezing temperature -48°C in the shortest possible time, the time is 120min ;
[0041] 2). Sublimation drying stage: When the vacuum degree in the drying oven reache...
Embodiment 3
[0044] prescription
[0045]
[0046] Preparation Process:
[0047] The mannitol, sodium bisulfite and sotalol hydrochloride of the prescribed amount are dissolved in water for injection accounting for 80% of the total volume, stirred and mixed evenly; the pH value is adjusted with an appropriate amount of pH value, and the volume is adjusted to the total volume with water for injection. Measure, stir and mix evenly; filter, fill in neutral borosilicate glass injection bottles according to 4ml / bottle, stopper halfway, freeze-dry, and visually inspect to get the finished product.
[0048] Freeze-drying curve:
[0049] 1). Pre-freezing stage: place the filled and semi-stoppered semi-finished product on the partition in the freeze-drying cabinet for pre-freezing, and make the semi-finished product reach the pre-freezing temperature in the shortest possible time. The pre-freezing temperature range is - 42℃, the time is 180min;
[0050] 2). Sublimation drying stage: When the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com