Pharmaceutical composition containing pokeberry antiviral proteins and immunologic active material and applications thereof
An immune active and antiviral technology, applied in the field of medicine, can solve problems such as long wound healing time, scab bleeding, cervical dilatation barriers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
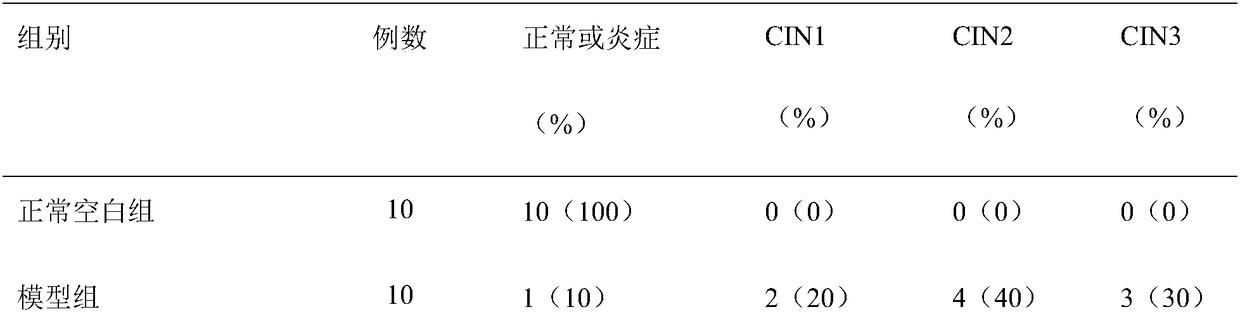
Embodiment 1
[0019] Pokeweed antiviral protein 0.1g, coconut oil 3g, Cordyceps extract 1g, Ganoderma lucidum extract 1g, polyinosinic acid-polycytidylic acid 1g, CMC-Na 3g, glycerin 10g, chlorhexidine 0.15g, purified water plus to 100g.
Embodiment 2
[0021] Pokeweed antiviral protein 0.5g, seaweed polysaccharide 2g, Ganoderma lucidum extract 0.1g, aloe vera gel powder 10g, polyinosinic acid-polycytidylic acid 0.1g, carbomer 1g, glycerin 10g, triethanolamine 1g, polysorbate 1 g of alcohol, 0.05 g of ethyl p-hydroxybenzoate, an appropriate amount of 95% ethanol, and purified water to 100 g.
Embodiment 3
[0023] Pokeweed antiviral protein 0.08g, Cordyceps extract 2g, Ganoderma lucidum extract 0.5g, propolis 1g, polyinosinic acid-polycytidylic acid 0.5g, carrageenan 6g, mix well, and make a compound agent with solvent before use.
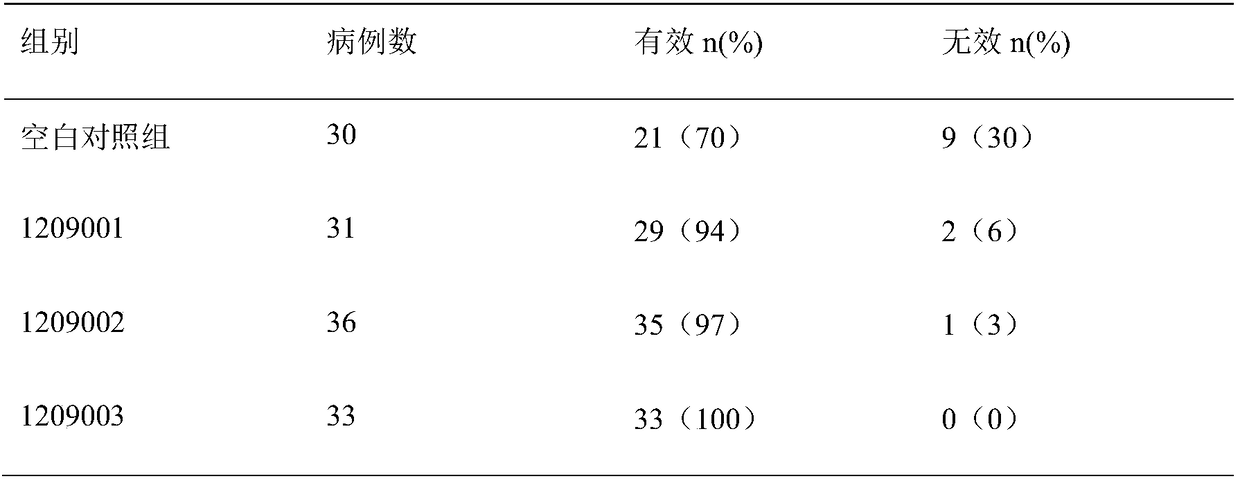
[0024] The batch numbers of the medicines prepared in the above three examples are 1209001, 1209002, and 1209003, and the preparation techniques of the three examples adopt conventional techniques.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com