Spiro compound used for treating cerebral apoplexy
A technology of compounds and hydrates, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0053] Hereinafter, the present invention is described in more detail to facilitate understanding of the present invention.
[0054] Those skilled in the art will recognize that the chemical reactions described herein can be used to suitably prepare many other compounds of the invention and that other methods for preparing the compounds of the invention are considered to be within the scope of the invention Inside. For example, the synthesis of those non-exemplified compounds according to the present invention can be successfully accomplished by those skilled in the art through modification methods, such as appropriate protection of interfering groups, by using other known reagents in addition to those described in the present invention, or by incorporating Reaction conditions with some routine modifications. In addition, reactions disclosed herein or known reaction conditions are also recognized to be applicable to the preparation of other compounds of this invention.
Embodiment 1
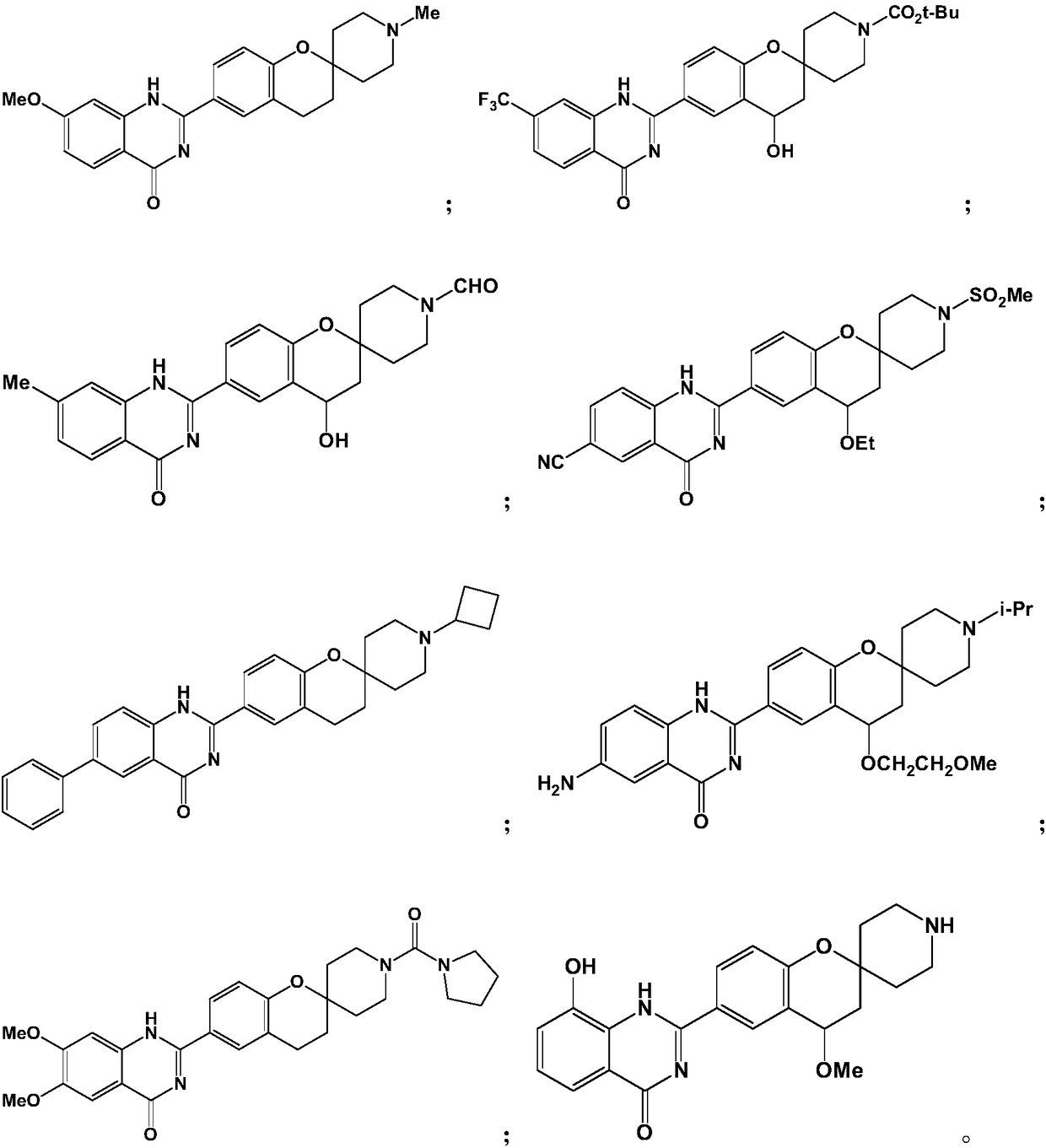
[0055] Example 1: 7-methoxy-2-(1'-methylspiro[chroman-2,4'-piperidin]-6-yl)quinazolin-4(1H)-one (SPQU-1)
[0056]
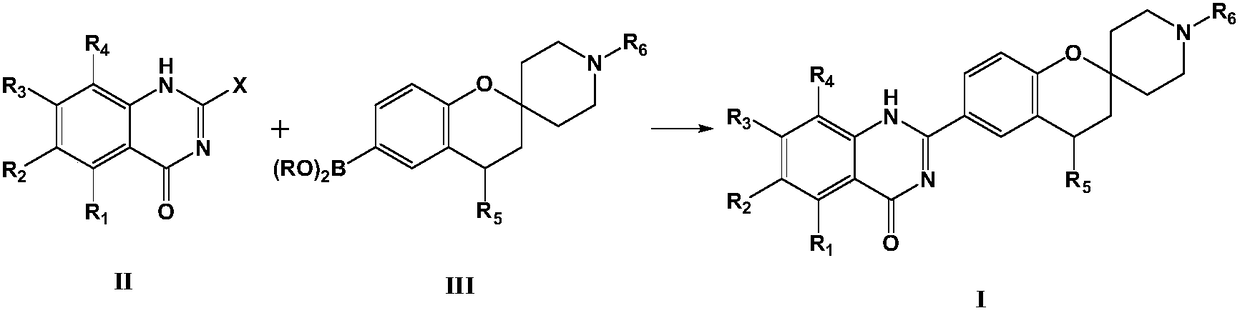
[0057] Add 50ml of dioxane to a 150ml flask, and add 2-bromo-7-methoxyquinazolin-4(1H)-one (2.0mmol) while stirring, 1'-methylspiro[chroman Pyran-2,4'-piperidine]-6-boronic acid pinacol ester (3.0mmol), then add Pd(dppf)Cl 2 (15 mg), potassium acetate (4.0 mmol). Under the protection of nitrogen, it was heated to 90° C. to react overnight. After filtration, the solvent was distilled off from the filtrate under reduced pressure, and the residue was purified by silica gel column (cyclohexane / ethyl acetate=10:1) to obtain 661 mg of the target compound, 84.6%.
[0058] Mass Spectrum (ESI): 392.19[M+H] +
[0059] Elemental analysis: theoretical value C, 70.57; H, 6.44; N, 10.73; O, 12.26
[0060] Found value C, 70.88; H, 6.35; N, 10.39; O, 12.38
[0061] Hydrogen spectrum (400MHz, DMSO) δ7.65(s,1H),7.54(d,1H),7.31(d,1H),7.09(d,1H),6.58(d,1H),6.30(s,1H) , 4....
Embodiment 2
[0062] Example 2: 4-hydroxyl-6-(4-oxo-7-(trifluoromethyl)-1,4-dihydroquinazolin-2-yl)spiro[chroman-2, 4'-piperidine]-1'-tert-butyl carboxylate (SPQU-2)
[0063]
[0064] Add 50ml of dioxane to a 150ml flask, and add 2-bromo-7-trifluoromethylquinazolin-4(1H)-one (2.0mmol) while stirring, 4-hydroxy-1'-tert-butoxy Carbonyl-spiro[chroman-2,4'-piperidine]-6-boronic acid pinacol ester (3.0mmol), then add Pd(dppf)Cl 2 (15 mg), potassium acetate (4.0 mmol). Under the protection of nitrogen, it was heated to 90° C. to react overnight. After filtration, the solvent was distilled off from the filtrate under reduced pressure, and the residue was purified by silica gel column (petroleum ether / ethyl acetate=10:1) to obtain 661 mg of the target compound, 84.6%.
[0065] Mass Spectrum (ESI): 532.20[M+H] +
[0066] Elemental analysis: theoretical value C, 61.01; H, 5.31; F, 10.72; N, 7.91; O, 15.05
[0067] Measured value C, 61.25; H, 5.08; F, 10.91; N, 7.83; O, 14.93
[0068] Proton...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com