Conductive polymer coated nickel-cobalt lithium aluminate cathode material and preparation method thereof
A conductive polymer, nickel cobalt lithium aluminate technology, applied in the direction of positive electrode, battery electrode, active material electrode, etc., can solve the problems of uneven coating, poor electrochemical performance, etc., to improve rate performance, reduce surface alkali performance, improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] A preparation method of a nickel-cobalt-aluminum positive electrode material coated with a conductive polymer, comprising the following steps:
[0024] (1) Add sodium dodecylbenzenesulfonate into deionized water, stir for a certain period of time to make it completely dissolve, and configure it into a solution of 0.002-0.01g / mL;
[0025] (2) lithium nickel cobalt oxide powder (LiNi x co y Al 1-x-y o 2 , x≥0.8, y≥0.1) are dispersed in the solution obtained in step (1), so that the mass ratio of lithium nickel cobaltate powder to sodium dodecylbenzenesulfonate is (10-20):1, and the conductive polymer is added monomer and oxidizing agent, in-situ polymerization at 0-10°C for 4-10h, the conductive polymer monomer is one of aniline, pyrrole and thiophene, and the oxidizing agent is (NH 4 ) 2 SO 8 、K 2 Cr 2 o 7 、KIO 3 , FeCl 3 , FeCl 4 、H 2 o 2 , Ce(SO 4 ) 2 , MnO 2 and a kind of in BPO (benzoyl peroxide), and the mass ratio of conductive polymer monomer and ...
Embodiment 1
[0029] Weigh 0.33 g of sodium dodecylbenzene sulfonate and add it into deionized water, stir for a certain period of time to completely dissolve it, and prepare a 0.01 g / mL solution. Take by weighing 5g lithium nickel cobalt oxide powder (LiNi 0.8 co 0.15 Al 0.05 o 2 ) was dispersed in the above solution, after the dispersion was uniform, 0.1 g of aniline and 0.245 g of ammonium persulfate were added, and in-situ polymerization was carried out at 0° C. for 10 h. The solution was filtered, washed with deionized water and ethanol several times, and dried in vacuum at 100° C. for 20 hours to obtain a polyaniline-coated nickel-cobalt-lithium-aluminate positive electrode material.
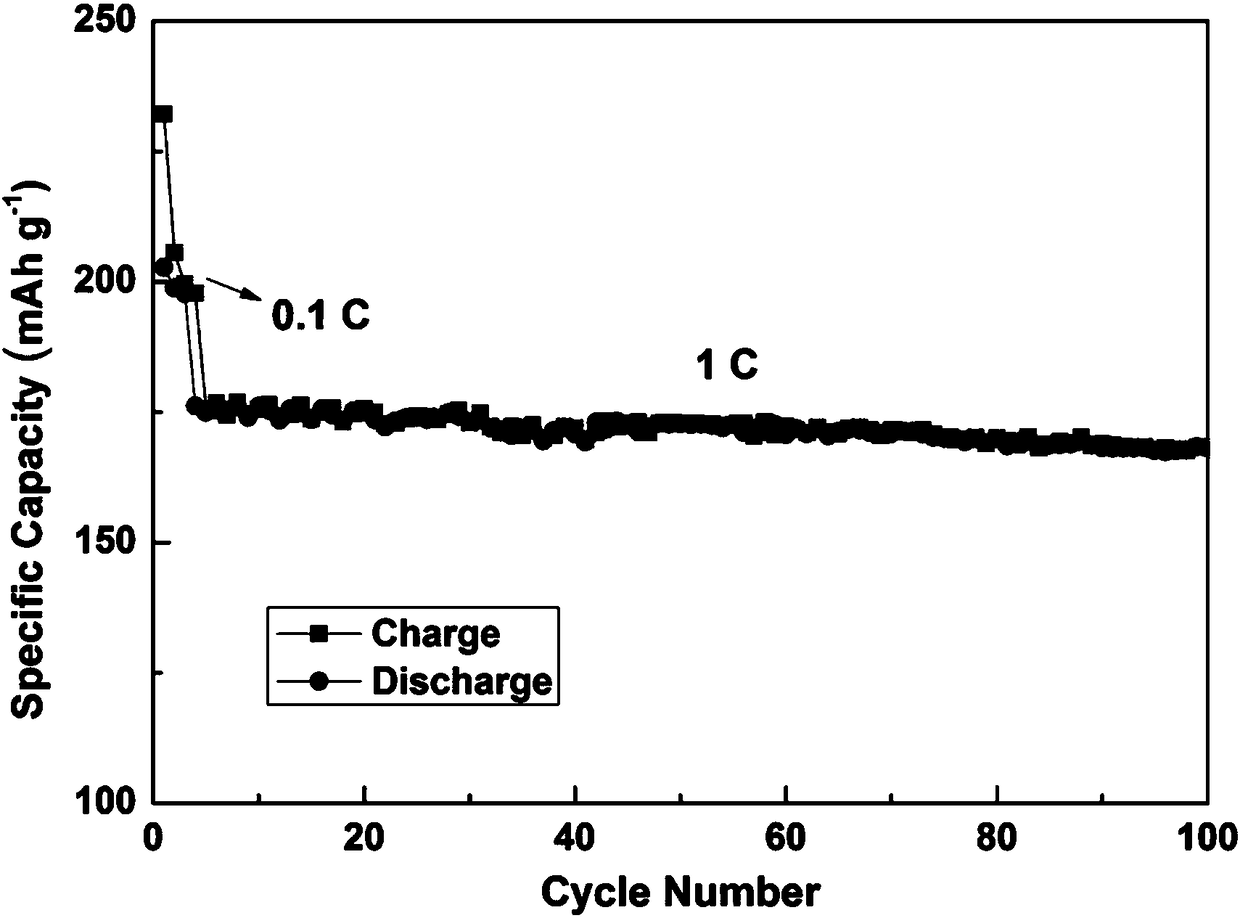
[0030] Coating the prepared positive electrode material into a positive electrode sheet, electrolyte, diaphragm, nickel mesh, and lithium sheet negative electrode were assembled into a button battery for testing. For test results see figure 1 , at 0.1C, the first discharge specific capacity is 203m...
Embodiment 2
[0032] Weigh 0.4 g of sodium dodecylbenzene sulfonate and add it into deionized water, stir for a certain period of time to dissolve it completely, and prepare a solution of 0.002 g / mL. Take by weighing 8g lithium nickel cobalt oxide powder (LiNi 0.8 co 0.15 Al 0.05 o 2 ) is dispersed in the above solution, after the dispersion is uniform, add 0.024g of pyrrole and 0.145g of FeCl 3 , In situ polymerization at 5°C for 4h. The solution was filtered, washed with deionized water and ethanol several times, and dried in vacuum at 100° C. for 20 hours to obtain a polypyrrole-coated nickel-cobalt-lithium-aluminate positive electrode material.
[0033] Coating the prepared positive electrode material into a positive electrode sheet, electrolyte, diaphragm, nickel mesh, and lithium sheet negative electrode were assembled into a button battery for testing. At 0.1C, the first discharge specific capacity is 206mAh g -1 , efficiency 89.3%, 1C cycle discharge 185mAhg in the first cycle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com