Additive for improving nickel anode catalyst performance of direct borohydride fuel cell
A fuel cell and nickel-based catalyst technology, applied in the field of electrochemical applications, can solve problems such as loss of catalytic activity, easy corrosion, and high resistance to charge transfer in electrode reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
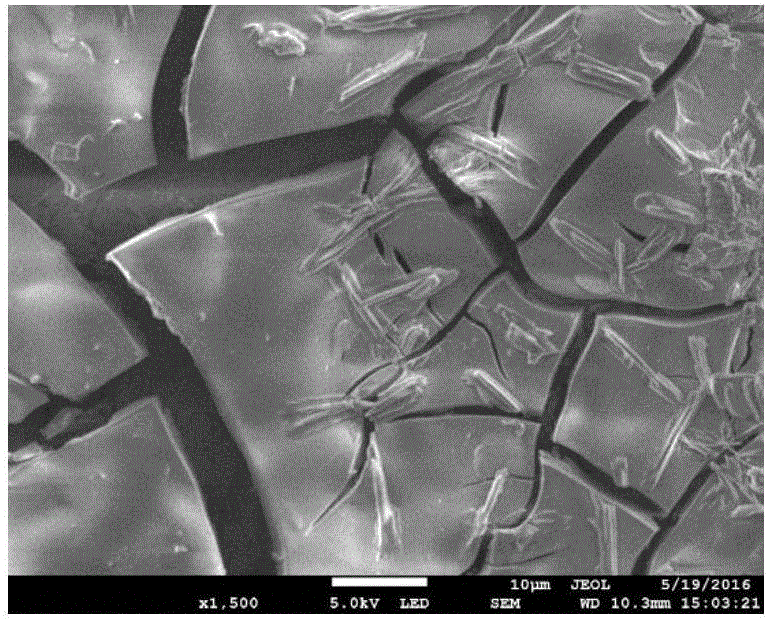
[0017] Under normal pressure, the temperature is in the range of 293.15 ~ 313.15K, with 0.2mol / L NiSO 4 solution. will be 2.0cm 2 The smooth Ni sheet (as the working electrode) is placed in the above solution, the smooth Ni sheet is used as the counter electrode, and the calomel electrode is used as the reference electrode, and the metal nickel is deposited on the Ni sheet electrode by the constant potential (-0.8V) method. into a nickel-based catalyst. Weigh an appropriate amount of sodium borohydride (NaBH 4 ), and dissolve it in 2.0mol / L sodium hydroxide (NaOH) solution to make 0.27mol / LNaBH 4 solution, mixed well as direct NaBH 4 Electrolyte for fuel cells. Configure 0.045mol / L thiourea solution, then take 1mL of prepared thiourea solution and dilute it 5 times to make the concentration of thiourea solution 0.009mol / L. Measure 30mL of electrolyte solution, add 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.1μL thiourea solution to it, 2 The nickel-based cata...
Embodiment 2
[0023] Under normal pressure, the temperature is in the range of 293.15 ~ 313.15K, with 0.2mol / L NiSO 4 solution. will be 2.0cm 2 The smooth Ni sheet (as the working electrode) is placed in the above solution, with the smooth Ni sheet as the counter electrode and the calomel electrode as the reference electrode, the metal nickel is deposited on the Ni sheet by the constant potential (-0.8V) method to make nickel base catalyst. Weigh an appropriate amount of NaBH 4 , and dissolve it in 2.0mol / L NaOH solution to make 0.27mol / LNaBH 4 solution, mixed well as direct NaBH 4 Electrolyte for fuel cells. Add 0.21μmol / L thiourea to the electrolyte solution, at 2.0cm 2 The nickel-based catalyst is used as the working electrode, the mercury / mercury oxide electrode is used as the reference electrode, and the graphite rod is used as the auxiliary electrode, and the AC impedance spectroscopy performance test is performed.
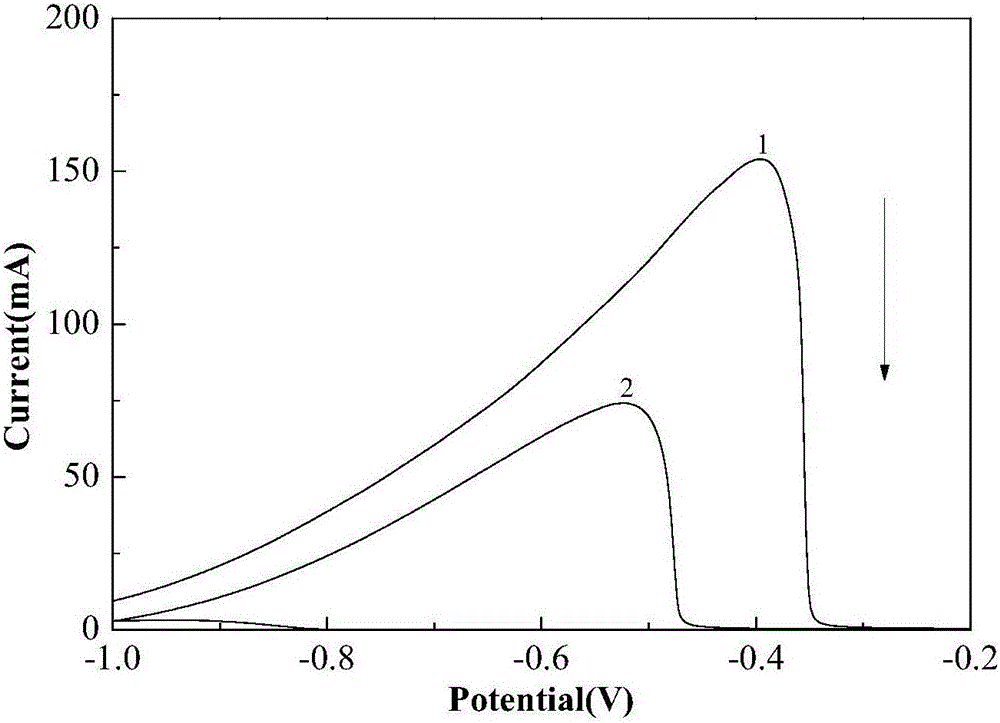
[0024] Figure 6 is BH before and after adding thiourea 4 ...
Embodiment 3
[0026] Under normal pressure, the temperature is in the range of 293.15 ~ 313.15K, with 0.2mol / L NiSO 4 solution. will be 2.0cm 2 The smooth Ni sheet (as the working electrode) is placed in the above solution, with the Ni sheet as the counter electrode and the calomel electrode as the reference electrode, the metal nickel is deposited on the Ni sheet by the constant potential (-0.8V) method to form a nickel base catalyst. Weigh an appropriate amount of NaBH 4 , and dissolve it in 2.0mol / L NaOH solution to make 0.27mol / LNaBH 4 solution, mixed well as direct NaBH 4 Electrolyte for fuel cells. Add 0.18μmol / L thiourea to the electrolyte solution, at 2.0cm 2 The deposited Ni catalyst was used as the working electrode, the mercury / mercury oxide electrode was used as the reference electrode, and the graphite rod was used as the auxiliary electrode, and the galvanostatic discharge performance test was performed.
[0027] Figure 7 is at a current density of 10mA / cm 2 Next, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com