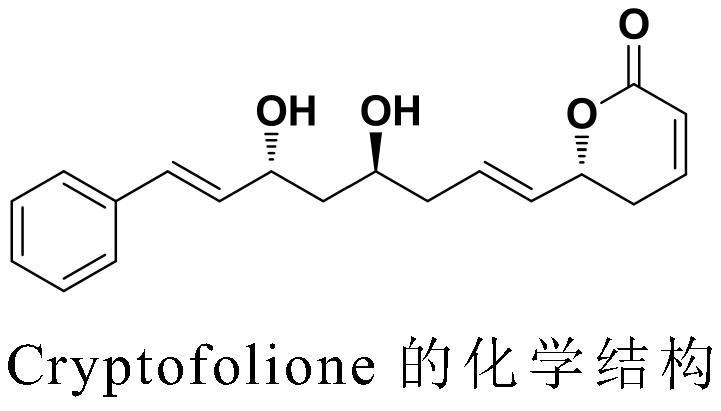
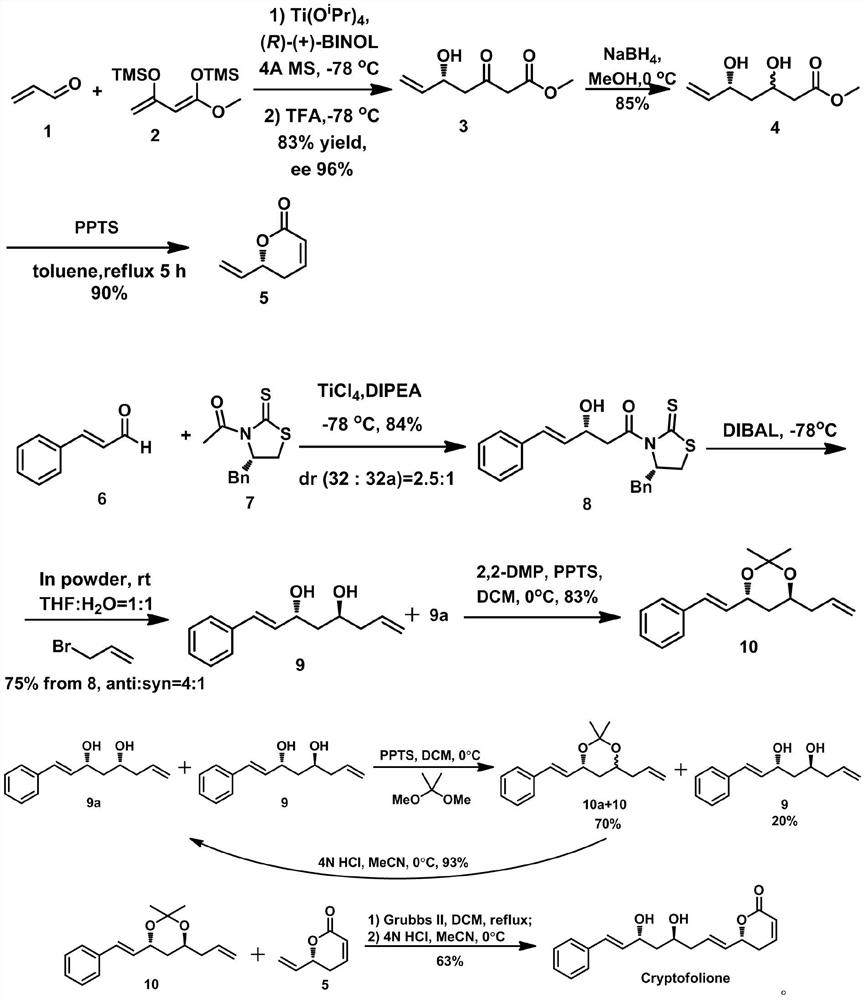
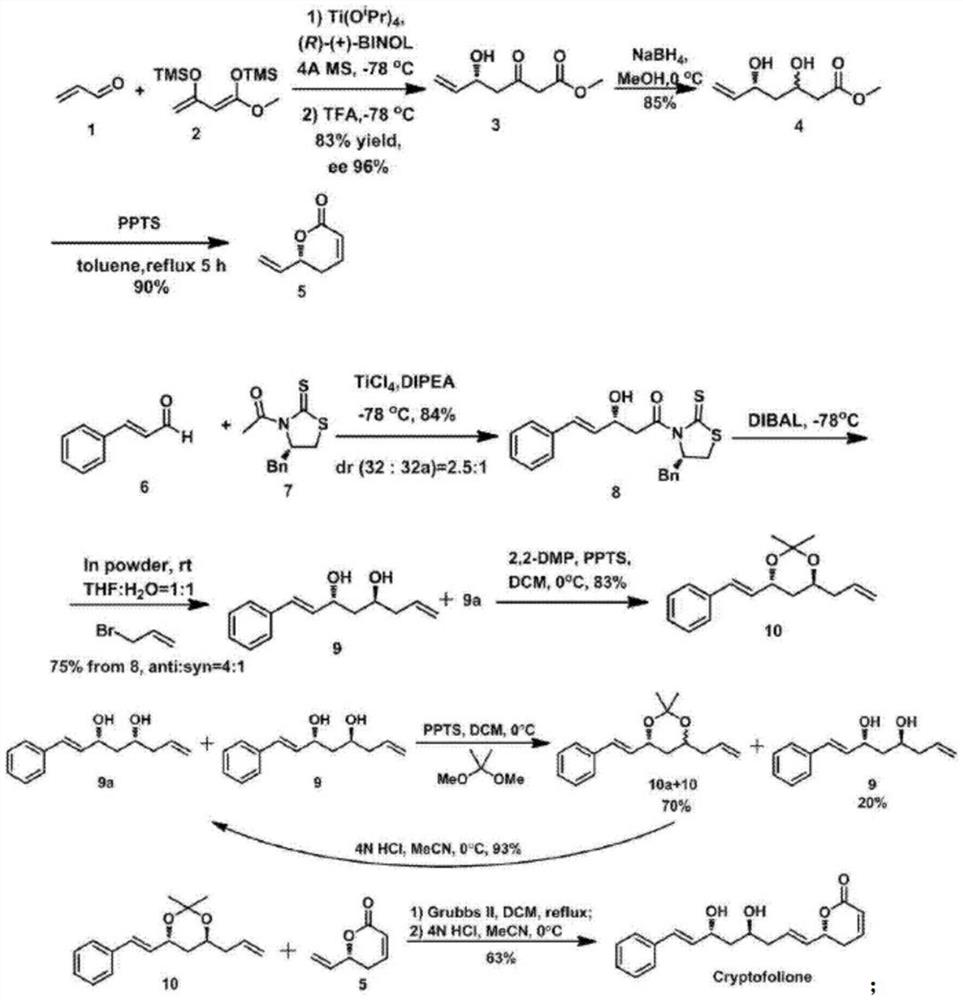
A kind of synthesis method of anti-trypanosome, anti-cancer natural product cryptofolione
A technology of natural products and synthetic methods, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of high synthesis cost and long route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Synthesis of the compound of formula 3: Weigh (R)-BINOL (0.14g, 0.48mmol) and (1.06g) was added to anhydrous tetrahydrofuran (1mL), and then Ti(O i Pr) 4 (70 μL, 0.24 mmol), and the resulting mixture was stirred at room temperature for 1 hour, and then the mixture was cooled to -78°C. A solution of acrolein (0.64 g, 3.03 mmol) in tetrahydrofuran was added dropwise. After reacting for 30 minutes, the compound of dienosilyl ether formula 2 (2.37 g, 9.09 mmol) was dissolved in tetrahydrofuran and introduced with a double-ended catheter. The resulting solution was stirred at -78°C under nitrogen for 2 hours, then transferred to room temperature and stirred overnight. After the reaction of acrolein was complete, the mixture was re-cooled to -78°C and trifluoroacetic acid (1.21 mL) was added dropwise. Warm to room temperature and continue stirring for 1 hour. It was then diluted with a small amount of ethyl acetate (5 mL), and saturated aqueous sodium bicarbonate (5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com