Targeted photosensitizer and preparation method and application thereof, and anti-tumor drug comprising targeted photosensitizer
An anti-tumor drug and photosensitizer technology, applied in the field of photosensitizers, can solve the problems of reduced photodynamic activity of photosensitizers, large molecular weight of photosensitizers, etc., and achieve the effects of good photodynamic activity, good targeting, and improving targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
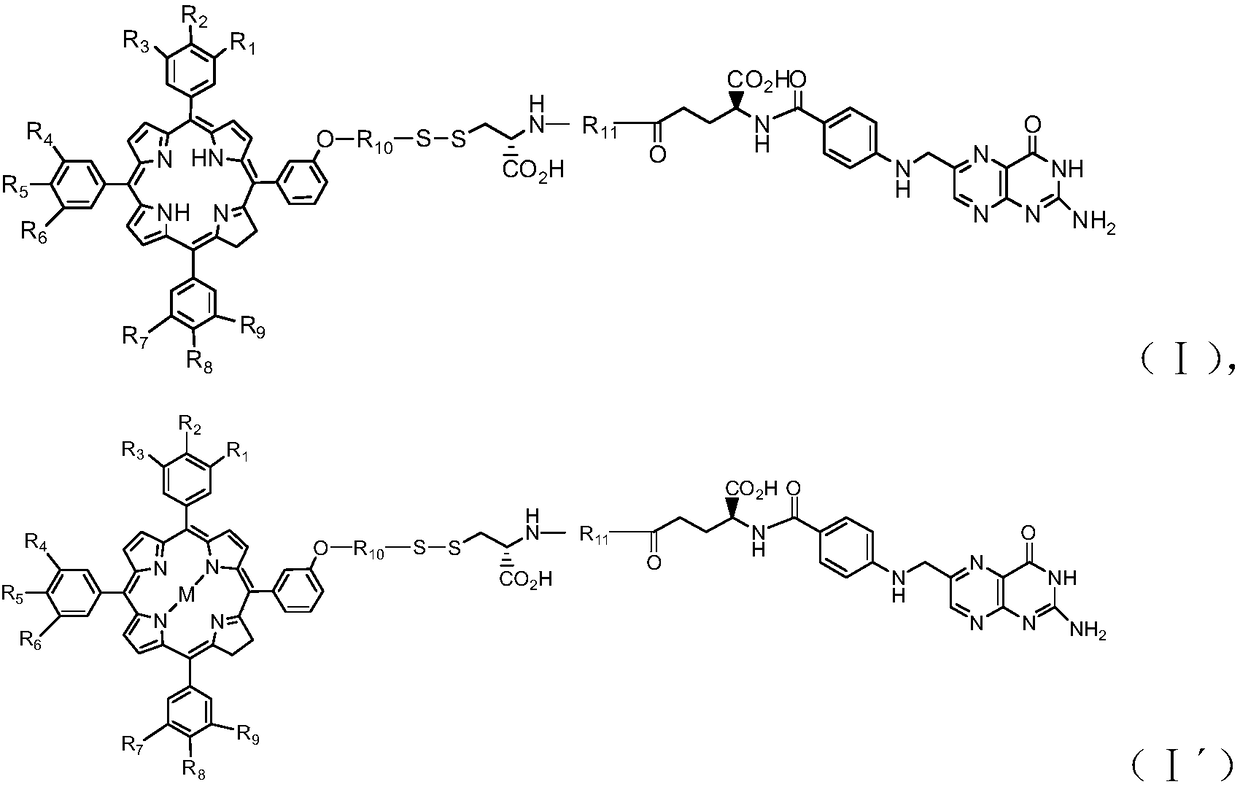
[0102] This embodiment provides a targeted photosensitizer, which is prepared according to the following steps:
[0103] 1) Preparation of photoactive effect molecular intermediates: Equimolar mTHPC, 2-(2-hydroxyalkyl)pyridine dithiopyridine and triphosgene were condensed in the dark, and then separated and purified by column chromatography to obtain carbonate bonds and bis Sulfur-bonded porphine intermediates;
[0104] 2) Preparation of cysteine-polypeptide: starting from Fmoc-arginine and Fmoc-cysteine,
[0105] Weigh the resin and place it in a solid-phase synthesis tube, add dichloromethane to swell, add 20%-40% pyridine-dichloromethane solution, after washing, add equimolar Fmoc-Arg and Fmoc-cysteine, the reaction bottom 2 times the condensing agent, the reaction temperature is room temperature; react for 10 hours, then alternately wash with dichloromethane and absolute ethanol until the by-products are washed clean; repeat the above steps to connect arginine in turn, th...
Embodiment 2
[0115] This embodiment provides a targeted photosensitizer. The difference from Example 1 is that in step 1), equimolar mTHPC, 2-(hydroxymethyl)pyridine dithiopyridine and equimolar triphosgene are used for the light-proof condensation reaction. ; In step 4), react under the condition of 60-80° C. for 6 hours to obtain the targeted photosensitizer.
Embodiment 3
[0117] This example provides a targeted photosensitizer, the difference from Example 1 is that the reaction products are not purified during the preparation of the targeted photosensitizer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com