Chlorpromazine tablet and preparation method thereof
A technology of chlorpromazine tablet and chlorpromazine hydrochloride, applied in the field of medicine, can solve the problems of low drug dissolution bioavailability, slow disintegration, burden and the like, and achieves convenient carrying and taking, high dissolution rate and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
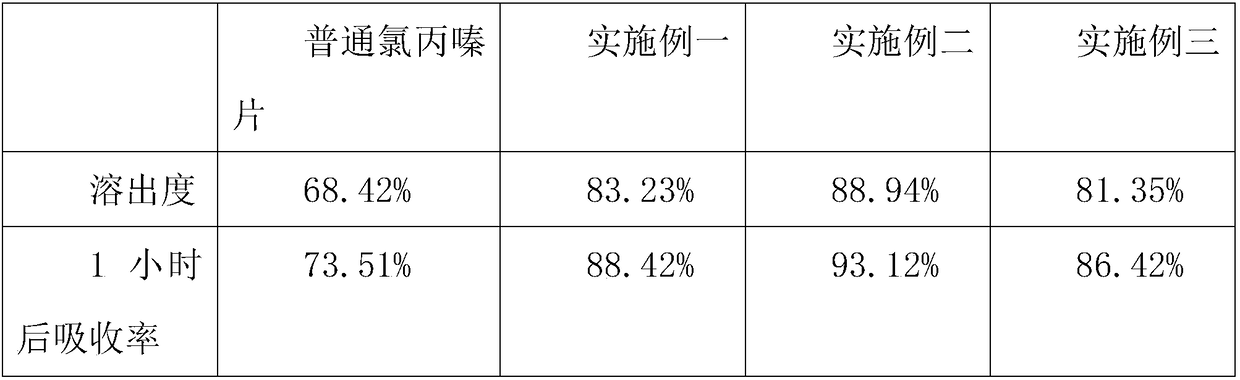
Embodiment example 1
[0028] A kind of chlorpromazine tablet, is made by the raw material of following weight ratio: 130 parts of lactose (314 types), 13 parts of cornstarch, 5 parts of povidone (K30), 18 parts of purified water, 28 parts of chlorpromazine hydrochloride 8 parts of carboxymethyl starch sodium (type A), 1 part of anhydrous silica gel, 1 part of magnesium stearate, 1 part of monkey mushroom extract, 5 parts of Opadry (21S58740), 44 parts of methanol, 65 parts of dichloromethane part, 0.3 part of antioxidant.
[0029] The antioxidant is stannous chloride.
[0030] The additive is one or more of talcum powder, cornstarch, and pharmaceutical grade cross-linked polyvinylpyrrolidone.
[0031] The water content of starch slurry is 10%-20%.
[0032] The preparation method of a kind of chlorpromazine sheet according to claim 1, is characterized in that: comprise the following steps:
[0033] (1), Lactose (type 314), cornstarch, and Hericium erinaceus extract are made into fillers using HZD...
Embodiment example 2
[0045] A kind of chlorpromazine sheet, is made by the raw material of following weight ratio: 135 parts of lactose (314 types), 15 parts of cornstarch, 6 parts of povidone (K30), 20 parts of purified water, 30 parts of chlorpromazine hydrochloride 10 parts of carboxymethyl starch sodium (type A), 2 parts of anhydrous silica gel, 2 parts of magnesium stearate, 2 parts of monkey mushroom extract, 6 parts of Opadry (21S58740), 45 parts of methanol, 66 parts of dichloromethane part, 0.4 part of antioxidant.
[0046] The antioxidant is stannous chloride.
[0047] The additive is one or more of talcum powder, cornstarch, and pharmaceutical grade cross-linked polyvinylpyrrolidone.
[0048] The water content of starch slurry is 10%-20%.
[0049] The preparation method of a kind of chlorpromazine sheet according to claim 1, is characterized in that: comprise the following steps:
[0050] (1), Lactose (type 314), cornstarch, and Hericium erinaceus extract are made into fillers using ...
Embodiment example 3
[0062] A kind of chlorpromazine tablet, is made by the raw material of following weight ratio: 140 parts of lactose (314 types), 7 parts of cornstarch, 7 parts of povidone (K30), 22 parts of purified water, 33 parts of chlorpromazine hydrochloride 12 parts of carboxymethyl starch sodium (type A), 3 parts of anhydrous silica gel, 4 parts of magnesium stearate, 3 parts of monkey mushroom extract, 7 parts of Opadry (21S58740), 46 parts of methanol, 68 parts of dichloromethane part, 0.5 part of antioxidant.
[0063] The antioxidant is stannous chloride.
[0064] The additive is one or more of talcum powder, cornstarch, and pharmaceutical grade cross-linked polyvinylpyrrolidone.
[0065] The water content of starch slurry is 10%-20%.
[0066] The preparation method of a kind of chlorpromazine sheet according to claim 1, is characterized in that: comprise the following steps:
[0067] (1), Lactose (type 314), cornstarch, and Hericium erinaceus extract are made into fillers using ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com