Sudden acute fulminating infectious disease emergency detection reserve kit and test method
An inspection method and emergency detection technology, which are applied in the determination/inspection of microorganisms, biochemical equipment and methods, and methods based on microorganisms, etc., can solve the problems of high storage cost, expensive storage of test kits, and prone to errors, etc. Achieve the effects of good specificity and stability, rapid emergency investigation, and extended storage time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
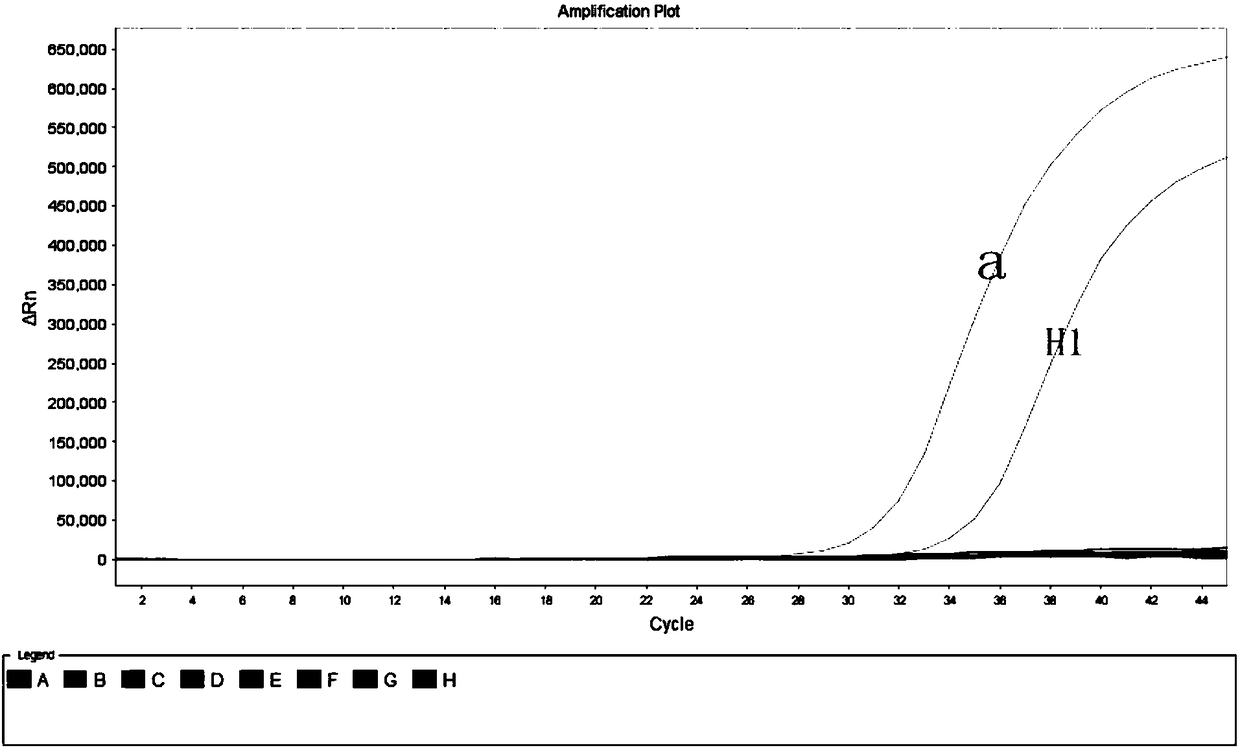
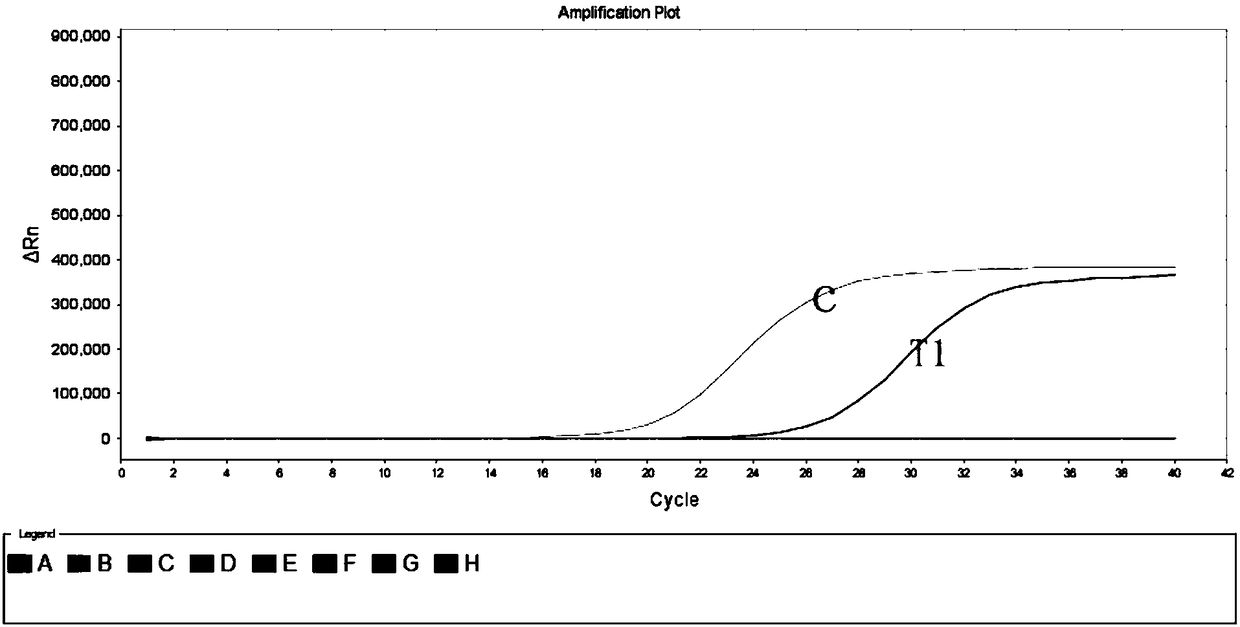
[0072] Example 1: Comparison of emergency reserve kits for sudden acute infectious diseases with commercial kits
[0073] 1. Sample preparation: Select human serum samples infected with dengue virus as DG1, influenza A virus cell culture fluid as H1 and Bacillus anthracis positive plasmid T1, and extract nucleic acids respectively. The 3 samples were tested using commercial influenza A virus fluorescent quantitative PCR detection kit, dengue virus fluorescent quantitative PCR detection kit, Bacillus anthracis fluorescent quantitative PCR detection kit and emergency reserve kit for sudden acute infectious diseases.
[0074] 2. PCR reaction system and reaction conditions: Configure the system and set the reaction conditions according to the instruction manual of the three kits, and at the same time take the influenza A F / R / P and dengue virus F in the emergency reserve kit for acute infectious diseases / R / P and anthrax F / R / P dry powder tubes were added to 230 μL nuclease-free wat...
Embodiment 2
[0082] Embodiment 2: unified reaction system and reaction condition
[0083] The kit contains more than 20 primers, probes and positive plasmids of terrorist factors, such as influenza virus, dengue virus, plague and so on. Taking influenza A virus positive plasmid as an example, the optimal reaction system and reaction conditions were found out. Use this reaction system and conditions to detect other terrorist factor-positive plasmids. The key reagent of fluorescent quantitative PCR is the probe, which is marked with different fluorescent groups, and the fluorescent markers of different pathogens are different. In this experiment, the probe markers of all pathogens are designed as 5'-FAM, 3'-BQ1, simplifying program setup process.
[0084] 1. System: Influenza virus positive plasmid is used as template, the concentration is 10ng / ml. Based on the reaction system of ABI one-step RT-PCR kit, explore the most suitable amount of F / R / P mixture. The list gradient is as follows: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com