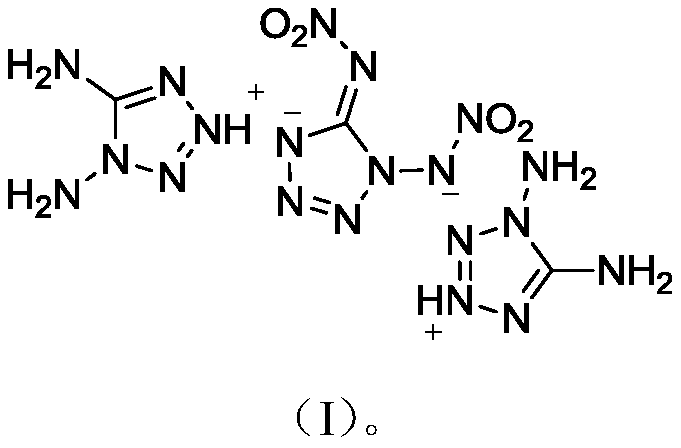
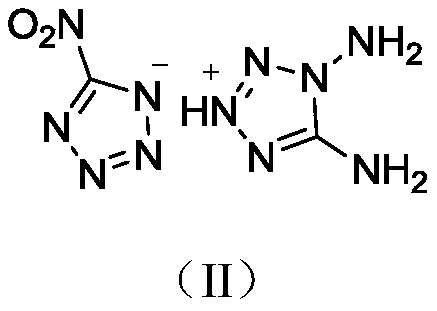
1,5-Dinitroaminotetrazole diaminotetrazolium salt compound
A technology of dinitroaminotetrazole diaminotetrazolium salt compound and structural formula, which is applied in the field of energetic materials and can solve the problems of low energy, low heat of formation, low density, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
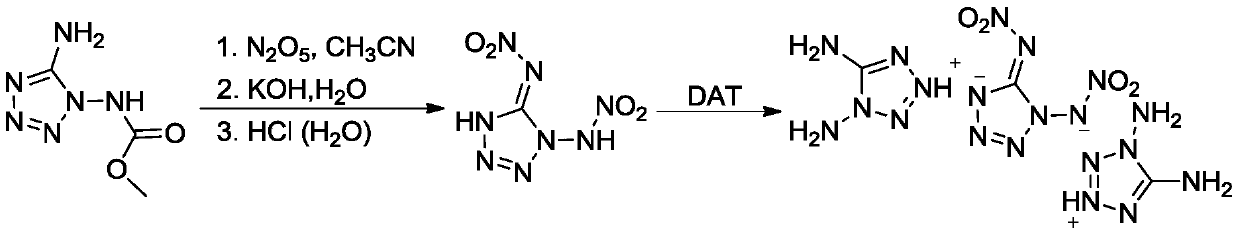
Embodiment 1
[0021] (1) Synthesis of 1,5-Dinitroaminotetrazole
[0022] At room temperature, add 2.21g (14mmol) of 1-methoxyformyl-1,5-diaminotetrazole into 42.0mL of acetonitrile, cool in an ice-water bath to 0-5°C, add dropwise 42.0mL containing 4.54g (42mmol) Dinitrogen pentoxide in acetonitrile solution, reacted at 0-5°C for 1 hour, continued to add 42.0 mL aqueous solution containing 4.70 g (84 mmol) of potassium hydroxide, reacted for 0.5 hour, distilled off the solvent to obtain a pale yellow solid, added 70.0 mL of methanol and stirred for 2 hours, Filter and dry to obtain a white solid; add 44.0mL of 2mol / L hydrochloric acid to the above white solid, stir for 0.5h, extract with ethyl acetate (40.0mL×4), dry over anhydrous magnesium sulfate, evaporate the solvent, and dry to obtain 2.11g of white Solid 1,5-dinitroaminotetrazole, yield 79.3%.
[0023] Structure Identification:
[0024] Infrared Spectrum: IR(KBr,cm -1 ), υ: 3205, 3144, 1596, 1579, 1508, 1476, 1453, 1413, 1332, 128...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com