A kit for studying ubiquitin-like system in bacteria and its application
A kit and ubiquitin-like technology, applied in the field of biology, can solve problems such as unclear reasons, poor expression and solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
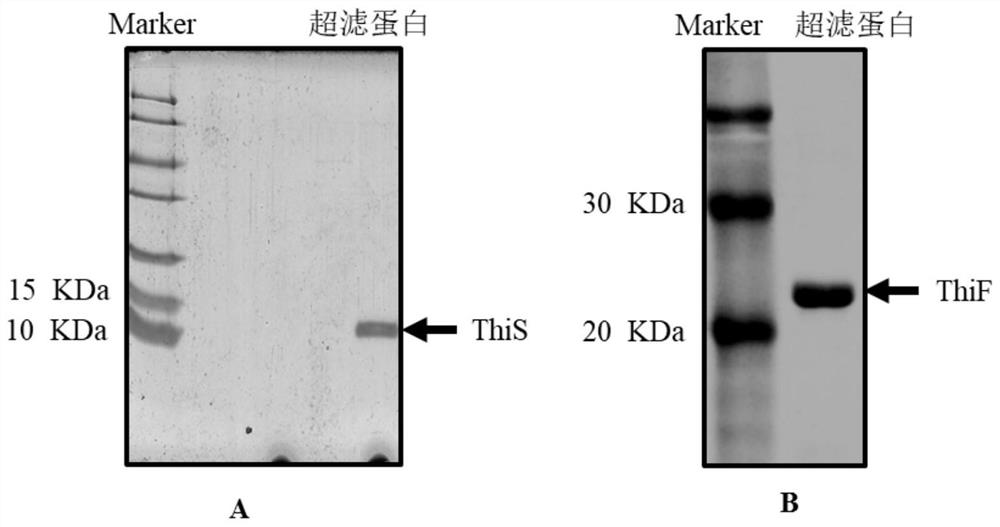
[0034] The kit for studying the ubiquitin-like system in bacteria in this example includes ThiS protein and ThiF protein, and the concentrations of ThiS protein and ThiF protein are 1 mg / mL respectively. The nucleotide sequence of ThiS protein is shown in SEQ ID NO.1, and the nucleotide sequence of ThiF protein is shown in SEQ ID NO.2. Align the amino acid sequence of ThiS protein with homologous protein sequences of other species, the results are as follows figure 1 As shown, ecol is the ThiS protein in this application, and the others are homologous sequences derived from Streptomyces and archaea. Through sequence comparison, it is found that ThiS has two very conserved glycines at the C-terminus, which are ubiquitin and ubiquitin-like Site of covalent attachment to substrate lysine.
[0035] The preparation steps of the ThiS protein and ThiF protein are as follows:
[0036] 1) Cloning the thiS gene and thiF gene into the commercial expression vector pET28-a respectively, ...
Embodiment 2
[0044] The kit for studying the ubiquitin-like system in bacteria in this example includes ThiS protein, ThiF protein, reaction buffer, DTT and ATP. ThiS protein and ThiF protein were purified by the method in Example 1, both at a concentration of 1 mg / ml. The reaction buffer includes MgCl 2 and Tris-HCl, Tris-HCl is 250mM pH 7.5, MgCl 2 It is 12.5mM in a bottle (named Reaction buffer). There is also a tube of 1M DTT and a tube of 100mM ATP. When in use, take 100 μl of Reaction buffer and add 0.5 μl of DTT to form a 5x buffer.
[0045] When the above kit is used: the mass concentration ratio of ThiS protein, ThiF protein and substrate protein is 4:2-4:1-4. The concentration of the ThiS protein is 0.15-0.25 μg / μL, preferably 0.02 μg / μL.
[0046] MgCl in the reaction buffer 2 The concentration used can be 2.3-2.7mmol / L, preferably 2.5mmol / L. The concentration of Tris-HCl can be 45-55mmol / L, preferably 50mmol / L, and the pH is 7.5. The concentration of ATP used can be 3.5-...
Embodiment 3
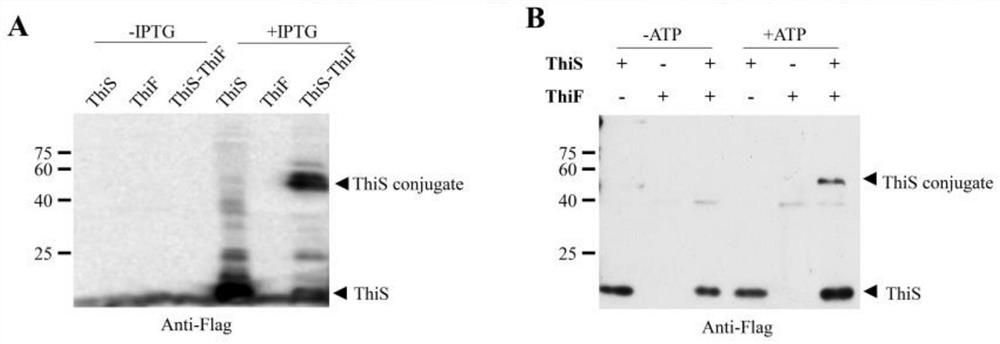
[0048] The ubiquitination reaction of ThiS protein and ThiF protein after overexpression in Escherichia coli.
[0049] With the pET28-a-thiS in embodiment 1 N-Flag Transform Escherichia coli Rosetta (DE3) with and / or pET28-a-thiF respectively, pick a single clone, culture in LB medium at 37°C overnight, dilute 1:100 into 1L of fresh LB medium, and culture until OD600 reaches 0.6-0.8 , adding (or not adding) the inducer IPTG with a final concentration of 0.5 mM, and induced overnight at 16°C. Collect the cells, add 2x SDS-PAGE loading buffer, heat at 95°C for 10 minutes to obtain a total protein sample, run SDS-PAGE gel (15%), without staining, use Anti-Flag mouse monoclonal antibody to incubate for 2 hours, after washing Then use the mouse secondary antibody to incubate for 1h, ECL exposure for 1min, the results are as follows: image 3 As shown in -A, it can be seen from the figure that after overexpressing ThiS protein and ThiF protein in Escherichia coli using IPTG induct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com