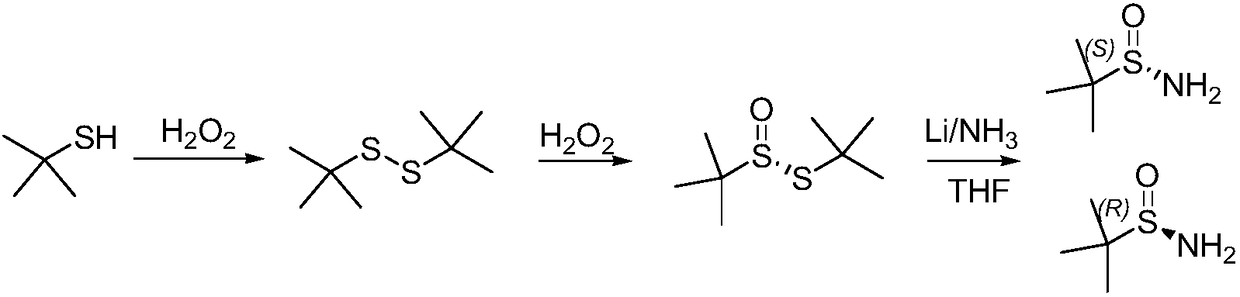
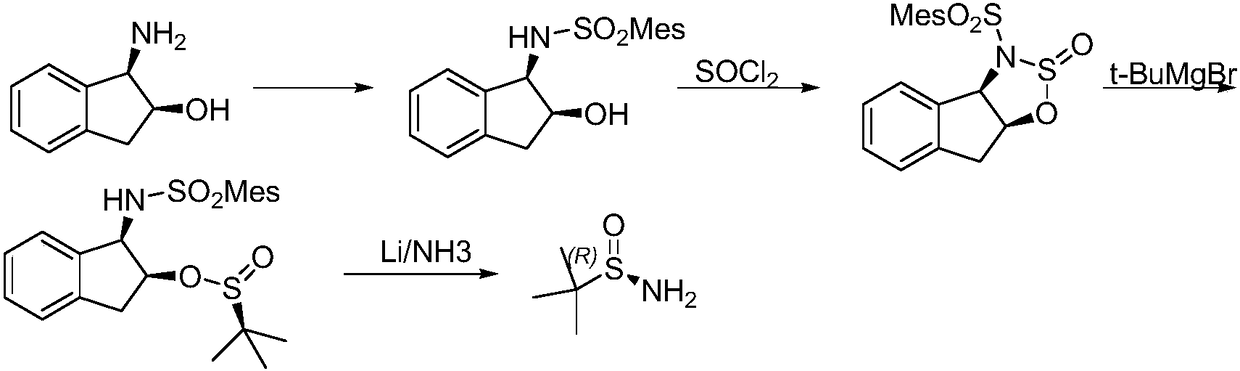
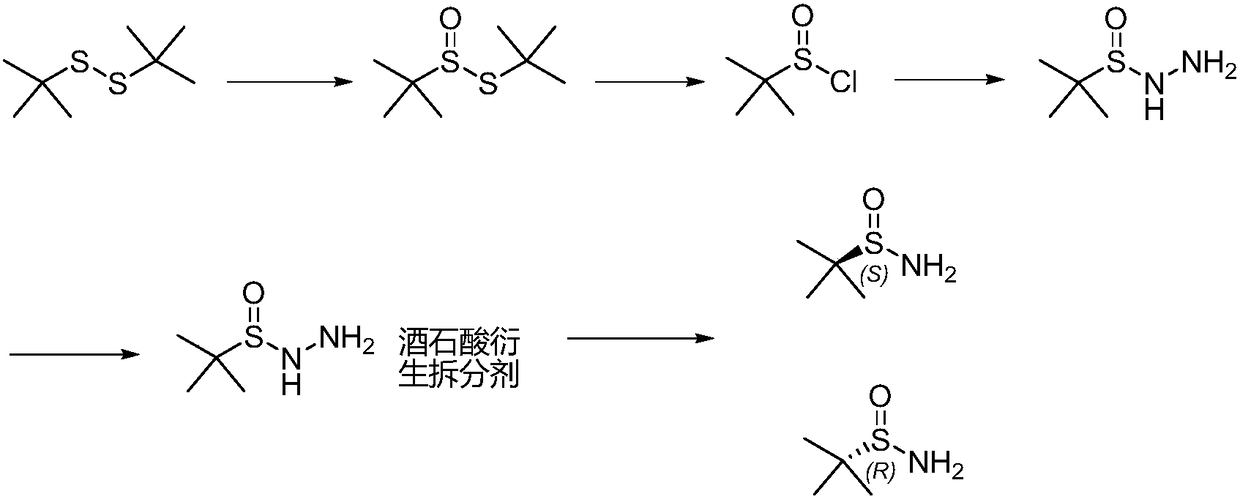
Method of synthesizing enantiomerically pure tert-butanesulfinamide
A technology of tert-butyl sulfinamide and tert-butyl sulfinyl, applied in the field of pharmaceutical synthesis, can solve the problems of high cost, long overall steps, unfavorable large-scale production, etc., and achieves reduced reaction time, simple operation, and convenient industrialization. Amplify the effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The first step: the synthesis of tert-butyl thiosulfinate.
[0043] (1) Add 180g tert-butyl disulfide, 1.8gVO(acac)2 and 400mL ethanol successively in the reaction bottle, add 138.5g 27% hydrogen peroxide dropwise at 25°C, react at 30-35°C for 7 hours, take a sample for TLC or According to HPLC detection, the raw material is less than 1.5%, concentrated under reduced pressure, washed once with saturated sodium chloride water, then added 400 g of dichloromethane to dilute sodium sulfate and dried for later use. The external standard yield was 88%.
[0044] (2) Add 180g of tert-butyl disulfide and 420mL of acetic acid in turn to the reaction bottle, add 150.1g of 27% hydrogen peroxide dropwise at 25°C, react at 30-35°C for 3 hours, take a sample for TLC or HPLC detection, and the raw material is less than 1% , then poured into 500g of water, extracted with dichloromethane, washed once with saturated sodium chloride water, dried over sodium sulfate for use. The external ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com