PCT detection kit based on bimolecular fluorescence complementation technology, preparation method and use method thereof
A detection kit and bimolecular technology, applied in the field of bimolecular fluorescence complementation, can solve the problems of large intra-batch variation, poor repeatability, and unstable reagents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
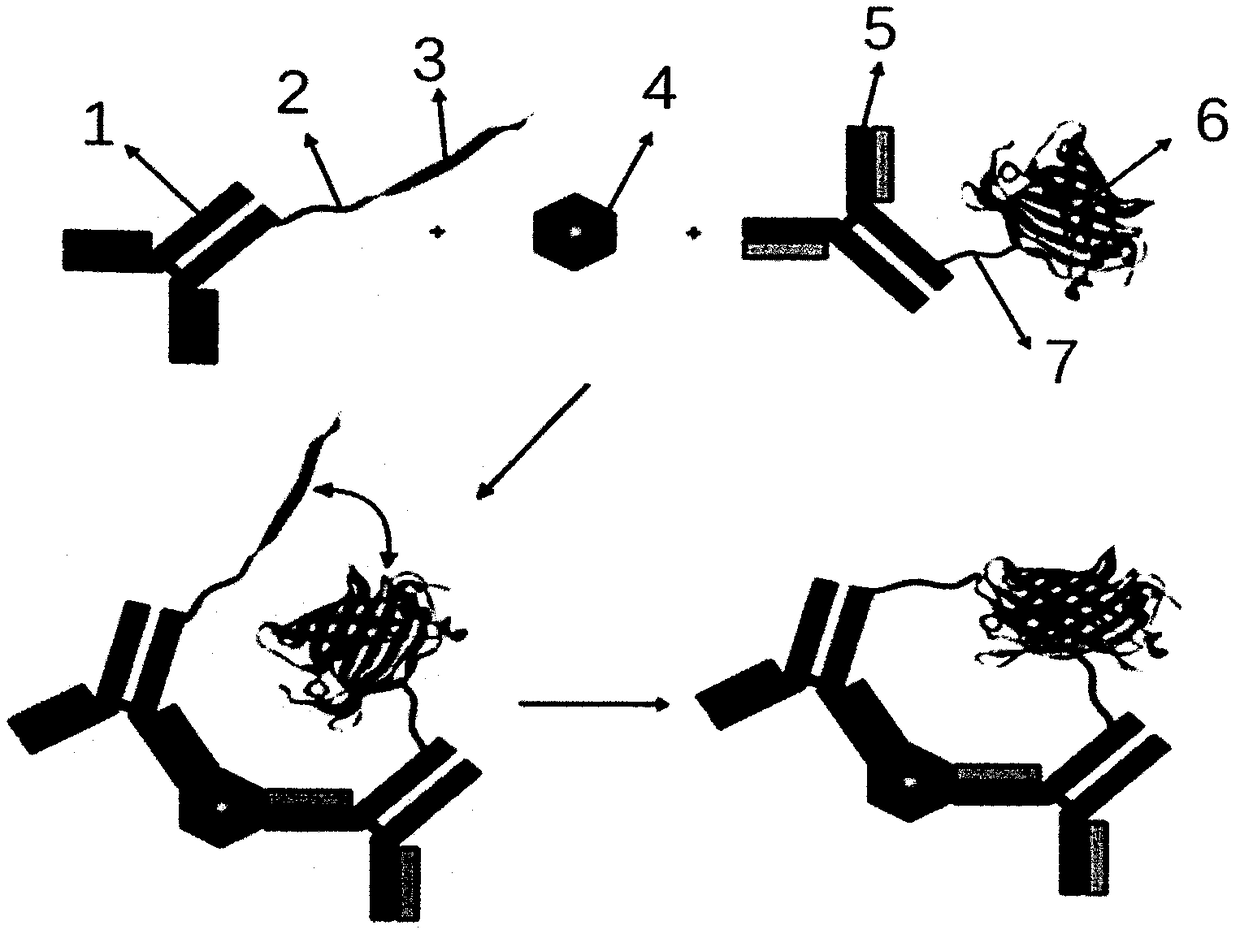
[0029] The anti-PCT antibody is coupled to the N-terminal fragment of fluorescent protein, taking the yellow fluorescent protein (YFP) 1-154 amino acid fragment YFPN as an example, the specific implementation process is as follows:
[0030] 1) Add 0.1mg of YFPN protein into a centrifuge tube, and prepare with 0.05mol / L pH9.5 carbonate buffer (CB) to dilute the YFPN protein to 1mg / ml.
[0031] 2) Add glutaraldehyde at a final concentration of 1.25% in a fume hood.
[0032] 3) Reaction in a water bath at 37°C for 2 hours.
[0033] 4) Dialyze with desalting column Sephadex G-25 or 0.05mol / L pH9.5 CB to remove excess glutaraldehyde.
[0034] 5) Take 0.1 mg anti-PCT antibody, prepare 1 mg / ml antibody with 0.05 mol / L pH9.5 CB, and mix the activated YFPN protein with the antibody.
[0035] 6) React overnight at 4°C.
[0036] 7) Blocking: add 50 μl of 0.2 mol / L lysine solution, and block for 2 hours at room temperature to block residual aldehyde groups and terminate the reaction. ...
Embodiment 2
[0040] The anti-PCT antibody is coupled to the fluorescent protein C-terminal fragment, taking the yellow fluorescent protein (YFP) 155-238 amino acid fragment YFPC as an example, the specific implementation process is as follows:
[0041] 1) Add 0.1mg of YFPC protein into a centrifuge tube and prepare with 0.05mol / L pH9.5 carbonate buffer (CB) to dilute the YFPC protein to 1mg / ml.
[0042] 2) Add glutaraldehyde at a final concentration of 1.25% in a fume hood.
[0043] 3) Reaction in a water bath at 37°C for 2 hours.
[0044] 4) Dialyze with desalting column Sephadex G-25 or 0.05mol / L pH9.5 CB to remove excess glutaraldehyde.
[0045] 5) Take 0.1 mg anti-PCT antibody, prepare 1 mg / ml antibody with 0.05 mol / L pH9.5 CB, and mix activated YFPC protein and antibody.
[0046] 6) React overnight at 4°C.
[0047]7) Blocking: add 50 μl of 0.2 mol / L lysine solution, and block for 2 hours at room temperature to block residual aldehyde groups and terminate the reaction.
[0048] 8) ...
Embodiment 3
[0051] The main components of the kit:
[0052] 1) N-terminal fragment of fluorescent protein coupled with anti-PCT antibody;
[0053] 2) Anti-PCT antibody-coupled fluorescent protein C-terminal fragment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Analytical sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap